Impact of the indigenous rotavirus vaccine Rotavac in the Universal Immunization Program in India during 2016-2020
- PMID: 41057654
- PMCID: PMC12618247
- DOI: 10.1038/s41591-025-03998-9
Impact of the indigenous rotavirus vaccine Rotavac in the Universal Immunization Program in India during 2016-2020
Erratum in
-
Author Correction: Impact of the indigenous rotavirus vaccine Rotavac in the Universal Immunization Program in India during 2016-2020.Nat Med. 2025 Nov;31(11):3931. doi: 10.1038/s41591-025-04062-2. Nat Med. 2025. PMID: 41145794 Free PMC article. No abstract available.
Abstract
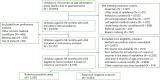
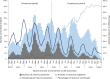
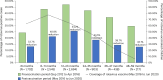
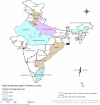
In 2016, India introduced Rotavac (G9P[11]), an indigenous oral rotavirus vaccine administered at 6, 10 and 14 weeks of age through the Universal Immunization Program. Evaluating its effectiveness under routine programmatic conditions is critical, given the variable performance of rotavirus vaccines in low- and middle-income countries. Here we assessed Rotavac's real-world effectiveness and impact across 31 hospitals in 9 states between 2016 and 2020 using a test-negative case-control design. Overall, 24,624 children were enrolled in surveillance (62% male and 38% female). Of 8,372 children aged 6-59 months eligible for effectiveness analysis (1,790 rotavirus-positive cases and 5,437 rotavirus-negative controls), 6,646 received 3 doses and 581 were unvaccinated. The adjusted vaccine effectiveness of 3 doses against severe rotavirus gastroenteritis was 54% (95% confidence interval (CI) 45% to 62%), with 1,574 vaccinated cases versus 5,072 vaccinated controls. Among children aged 6-23 months (1,486 vaccinated cases and 4,595 vaccinated controls), genotype-specific adjusted vaccine effectiveness was 51% (95% CI 36% to 62%) for G3P[8], 81% (95% CI 73% to 87%) for G1P[8] and 64% (95% CI 21% to 83%) for G1P[6]. Following vaccine introduction, rotavirus positivity among hospitalized children declined from 40% to 20%. These findings confirm that Rotavac provides substantial protection against severe rotavirus disease, including nonvaccine strains, and performs comparably to internationally licensed vaccines in similar settings.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.P.N. is the recipient of a fellowship from the Indian Council of Medical Research, New Delhi. G.K. is now an employee of the Bill and Melinda Gates Foundation. The other authors declare no competing interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The funders had no role in study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data from the study and had final responsibility for the decision to submit for publication. No authors were affiliated with Bharat Biotech.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

