Endothelial cell-derived apoptotic bodies modulate innate and adaptive immune responses during inflammation
- PMID: 41057874
- PMCID: PMC12502297
- DOI: 10.1186/s12964-025-02382-x
Endothelial cell-derived apoptotic bodies modulate innate and adaptive immune responses during inflammation
Abstract
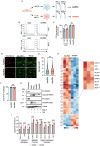
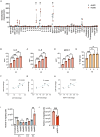
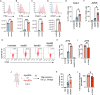
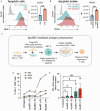
Endothelial cells (ECs) act as gatekeepers and signalling hubs that coordinate communication between blood vessels and surrounding tissues by regulating vascular tone, immune responses and numerous other physiological processes. During vascular inflammation commonly associated with aging, atherosclerosis, diabetes and autoimmunity, a range of biological, environmental and physical stressors can induce activation and apoptosis of ECs. Apoptotic bodies (ApoBDs) are large (~ 1-5 μm), membrane‑bound extracellular vesicles generated solely through apoptotic cell disassembly, that are increasingly recognised as mediators of intercellular communication via the transfer of bioactive molecules to target cells. Although EC apoptosis is a central feature of vascular inflammatory disorders, the formation of EC‑derived ApoBDs and their immunomodulatory roles when formed in an inflammatory environment, remains poorly defined. This study aimed to characterise the functional properties of EC‑derived ApoBDs generated under inflammatory conditions in vitro. A proteomics analysis of EC‑derived ApoBDs revealed that EC‑ApoBDs generated during inflammation ('iApoBDs') were enriched in inflammatory cytokines/chemokines, adhesion molecules and antigen presentation machinery compared with non-inflammatory ('ApoBD') controls. Functionally, iApoBDs promoted monocyte chemotaxis via the release of MCP-1, while altered expression of the adhesion molecule ICAM-1 enhanced efferocytosis by macrophages in vitro and in vivo. Furthermore, iApoBDs generated from antigen-pulsed HUVECs promoted IFN‑𝛾 expression by peptide specific CD8 T cells in an in vitro model of antigen presentation. These findings demonstrate that within an inflammatory setting, apoptotic ECs can participate in continued communication with their environment via the generation of ApoBDs, thereby modulating innate and adaptive immune processes. The formation of ApoBDs by ECs may serve as a target for therapeutic interventions in inflammatory vascular diseases.
Keywords: Apoptosis; Apoptotic bodies; Efferocytosis; Endothelial cells; Extracellular vesicles; Inflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Li Y, et al. Dynamics of endothelial cell generation and turnover in arteries during homeostasis and diseases. Circulation. 2024;149:135–54. - PubMed
-
- Rajagopalan S, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–83. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

