Long-term patient-derived ovarian cancer organoids closely recapitulate tumor of origin and clinical response
- PMID: 41057919
- PMCID: PMC12502492
- DOI: 10.1186/s13046-025-03537-x
Long-term patient-derived ovarian cancer organoids closely recapitulate tumor of origin and clinical response
Abstract
Background: Ovarian cancers are the second cause of death from gynecological cancers worldwide, due to a late diagnosis combined with the development of resistance to chemotherapy. However, half of these cancers present alterations in Homologous Recombination (HR), making them sensitive to inhibitors of the PARP protein (PARPi), involved in DNA repair. Nevertheless, identifying patients who respond to chemotherapy and selecting those eligible for PARPi remains a challenge for clinicians. In this context, the use of Patient-Derived Tumor Organoids (PDTO) for predictive functional testing represents an interesting prospect for clinical decision making.
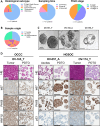
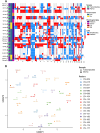
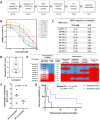
Methods: Here we established a panel of 37 long-term PDTO models of various histological subtypes from 31 ovarian cancer patients. Histological and molecular profiles of PDTO were compared to tumor sample of origin using immunohistochemical analyses and global approaches (copy number variation and transcriptomic profiling). PDTO models were exposed to standard drugs for ovarian cancer patients, including PARPi, and response was assessed using viability assay. To further define the HR status of PDTO, we performed a functional assay evaluating the ability of PDTO to initiate HR (RECAP test) using automated histo-imaging quantitative analysis of RAD51 foci, as well as an NGS analysis based on the sequencing of an HR-related genes panel to obtain a Genome Instability Score (GIS).
Results: We demonstrated that PDTO mimicked histological and expression of tumor markers of paired tumors. Moreover, non-negative matrix factorization approach revealed that PDTO recapitulated the transcriptomic profile of the cancer component from their sample of origin. Screening of chemotherapeutic drugs showed that PDTO exhibit heterogeneous responses, and that response of PDTO from high-grade serous ovarian carcinoma to carboplatin recapitulated patient response to first-line treatment. Additionally, the detection of HRD phenotype of PDTO using functional assay was associated with the results of the HRD test Genomic Instability Scar (GIScar).
Conclusion: Although larger-scale investigations are needed to confirm the predictive potential of PDTO, these results provide further evidence of the potential interest of ovarian PDTO for functional precision medicine.
Keywords: Functional assay; Homologous recombination; Ovarian cancer; Patient-derived tumor organoids.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consent forms were signed by all patients and were obtained either by the Biological Resources Center ‘OvaRessources’, which has received NF 96 900 accreditation (N° 2016/72860.1) or in the context of the ‘OVAREX’ clinical trial (N°ID-RCB: 2018-A02152-53, NCT03831230) [33], in accordance with ethical committee and European law. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304. - PubMed
-
- Gonzalez-Martin A, Harter P, Leary A, Lorusso D, Miller RE, Pothuri B, Ray-Coquard I, Tan DSP, Bellet E, Oaknin A, et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(10):833–48. - PubMed
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

