Safety, tolerability, immunogenicity, and efficacy of ABvac40 active immunotherapy against Aβ40 in patients with mild cognitive impairment or very mild Alzheimer's disease: A randomized, double-blind, placebo-controlled phase 2 study
- PMID: 41058018
- PMCID: PMC12504058
- DOI: 10.1002/alz.70776
Safety, tolerability, immunogenicity, and efficacy of ABvac40 active immunotherapy against Aβ40 in patients with mild cognitive impairment or very mild Alzheimer's disease: A randomized, double-blind, placebo-controlled phase 2 study
Abstract
Introduction: ABvac40 is an investigational active immunotherapy (vaccine) targeting Aβ40. This study assessed the safety and immunogenicity of ABvac40 in patients with amnestic mild cognitive impairment or very mild Alzheimer's disease.
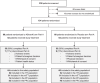
Methods: AB1601 was a multicenter, randomized, double-blind, placebo-controlled phase 2 study. Patients (n = 124) received five monthly injections plus a 10-month booster of ABvac40 or placebo, with 18-24 months of follow-up. Primary endpoints included safety, tolerability, and immunogenicity. Secondary endpoints assessed immune response, neuropsychological changes, and disease biomarkers.
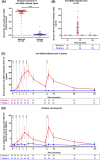
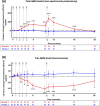
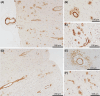
Results: Treatment-emergent adverse events (TEAEs) and serious TEAEs were comparable between ABvac40 (90.6% and 26.6%) and placebo (93.3% and 26.7%). Amyloid-related imaging abnormalities-hemorrhage (ARIA-H) were similar (12.5% ABvac40; 15.0% placebo), with no ARIA-edema (ARIA-E) or meningoencephalomyelitis. ABvac40 induced a specific, sustained immune response in plasma, with detectable antibodies in CSF.
Discussion: These findings support further investigation of ABvac40 as a potential disease-modifying therapy.
Clinical trial registration number: NCT03461276 (ClinicalTrials.gov) HIGHLIGHTS: ABvac40 was safe and well-tolerated in early-stage Alzheimer's disease patients. No amyloid-related imaging abnormalities-edema (ARIA-E) or encephalitis observed; ARIA-hemorrhage (ARIA-H) rates were similar across groups. Specific, sustained immune response to ABvac40 in plasma, with cerebrospinal fluid (CSF) antibody penetration. Cognitive scales and magnetic resonance imaging (MRI) volumetric data favored ABvac40 over placebo. Results support further development of ABvac40 as a disease-modifying therapy.
Keywords: ABvac40; Alzheimer's disease; Aβ40; active immunotherapy; amyloid‐β 40; cerebral amyloid angiopathy; clinical trial; disease‐modifying therapy; phase 2; randomized trial; vaccine.
© 2025 Araclon Biotech and The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M.P.L., A.M.L., M.M., J.C., J.L., I.M., J.A.A., L.S., N.F., and J.R. are full‐time employees of Araclon Biotech‐Grifols. MS was a full‐time employee of Araclon Biotech–Grifols, held several patents related to Alzheimer's disease diagnosis and treatment, and was the founder and a shareholder of Araclon Biotech–Grifols. M.T., D.W., and J.T. are full‐time employees of Grifols. G.P.R. has received consultancy fees, honoraria for lectures, and/or participated in advisory boards from the following companies: Grifols, Araclon Biotech, Lilly, Almirall, Nutricia, Schwabe Pharma, and Esteve. M.B. has received consultancy fees, honoraria for lectures, and/or participated in advisory boards from the following companies: Grifols, Araclon Biotech, Roche, Biogen, Lilly, Merck, Novo Nordisk, Bioiberica, Eisai, Servier, Schwabe Pharma, Nutricia, and Terumo. M.B. has also obtained research funding from Life Molecular Imaging, Bioiberica, Grifols, Araclon Biotech, Lilly, Roche, Janssen, Alzehon, Cortyzime, Novo Nordisk, and Schwabe Pharma. Author disclosures are available in the supporting Information.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

