Myoblast Choreographic Alignment
- PMID: 41058136
- PMCID: PMC12548339
- DOI: 10.1021/acsnano.5c08392
Myoblast Choreographic Alignment
Abstract
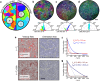
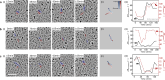
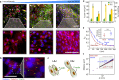
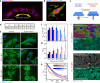
The alignment of spindle-shaped cells is crucial for physiological processes, such as muscle regeneration and tissue engineering. Despite their importance, the underlying mechanisms that govern this alignment remain poorly understood. In this study, we explore the collective motion and alignment of C2C12 myoblasts, cells essential for muscle regeneration, across a range of cell densities and substrate stiffness. Contrary to the conventional view that C2C12 cells exhibit weak cell-cell adhesion, leading to uncoordinated motion, our study shows that these cells demonstrate anisotropic adhesion properties. Specifically, cells connected longitudinally by nanoscale focal adherens junctions (FAJs) exhibit strong adhesion, whereas those connected laterally by nanoscale linear adherens junctions (LAJs) display weak adhesion. The interplay between these molecular junctions facilitates spontaneous alignment, even beyond confluence, contributing to the formation of multinucleated myotubes. Moreover, we show that softer substrates reduce the level of C2C12 cell alignment, emphasizing the role of extracellular environments in regulating cell behavior. These insights into the alignment dynamics of C2C12 myoblasts advance our understanding of contractile cell behavior and offer valuable contributions to the fields of muscle regeneration, tissue engineering, and cellular biomechanics.
Keywords: adherens junctions; cell morphogenesis; correlation length; extracellular interactions; soft matter.
Figures


References
-
- Vicsek T., Zafeiris A.. Collective motion. Phys. Rep. 2012;517:71–140. doi: 10.1016/j.physrep.2012.03.004. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

