Biochemical and Biological Profiles of Bangladeshi Russell's Viper Snake Venom and Neutralizing Efficacy by Indian VINS Polyvalent Antivenom
- PMID: 41058971
- PMCID: PMC12500362
- DOI: 10.1155/jt/5464388
Biochemical and Biological Profiles of Bangladeshi Russell's Viper Snake Venom and Neutralizing Efficacy by Indian VINS Polyvalent Antivenom
Abstract
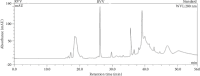
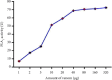
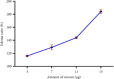
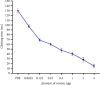
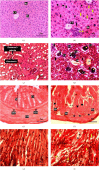
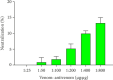
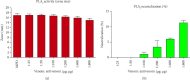
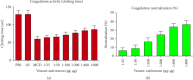
The Russell's viper (Daboia russelii) has recently become a significant threat to human life in Bangladesh. Given its wide distribution across South Asia, the venom characteristics and lethality can vary by region with different toxicological properties. Hence, we investigated the characteristics of Bangladeshi Russell's viper venom (BRVV) through SDS-PAGE profiling, reverse-phase HPLC analysis, along with assessments of phospholipase A2 (PLA2), edema-inducing, hemolytic, hemorrhagic, and coagulant activities, histopathology, and blood biochemistry, following established protocols. We also studied the neutralization efficacy of polyvalent antivenom from VINS Bio Products Ltd., India (VPAV) against BRVV. RP-HPLC analysis of BRVV displayed 15 peaks, and SDS-PAGE showed high-intensity protein bands within the 15-70 kDa range. The median lethal dose (LD50) for mice was found to be 0.33 mg/kg intraperitoneally (i.p.), and venom exposure resulted in neurotoxic symptoms such as limb paralysis, respiratory difficulties, and sluggishness. BRVV exhibited strong PLA2, procoagulant, hemorrhagic, indirect hemolytic, and edema-inducing activities but poor direct hemolytic activity. Venom administration also significantly increased levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), cholesterol, total protein, uric acid, blood urea nitrogen (BUN), and creatinine in mouse serum, indicating organ damage. Histopathological examination revealed cell vacuolization, congestion, hemorrhage, inflammatory infiltrations, and necrosis in venom-exposed tissues, validating the abnormal serum biochemistry. The neutralization study revealed that VPAV had limited efficacy against BRVV, suggesting the presence of venom proteins not fully neutralized by the antivenom. Altogether, these findings suggest that the Russell's viper is a medically significant venomous snake in Bangladesh, and VPAV is only partially effective in reducing the venom's toxic effects. Therefore, region-specific venoms must be considered in antivenom development for more effective treatment in envenomation cases.
Keywords: Bangladeshi Russell's viper; PLA2; VINS polyvalent antivenom; hemorrhage; histopathology; procoagulant.
Copyright © 2025 Rubait Hasan et al. Journal of Toxicology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Who. Guidelines for the Production, Control and Regulation of Antivenom Immunoglobulins . WHO; 2010. - PubMed
-
- Hossain J., Biswas A., Rahman F., Mashreky S. R., Dalal K., Rahman A. Snakebite Epidemiology in Bangladesh: a National Community Based Health and Injury Survey. In Health . 2016;08(05):479–486. doi: 10.4236/health.2016.85051. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

