Corrosion inhibition mechanisms of metal-organic frameworks in ammonia-rich environments
- PMID: 41059048
- PMCID: PMC12498158
- DOI: 10.3389/fchem.2025.1644300
Corrosion inhibition mechanisms of metal-organic frameworks in ammonia-rich environments
Abstract
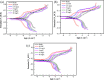
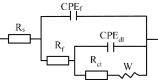
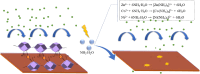
A range of metal-organic framework (MOF)-based composite materials were synthesized and assessed for their corrosion inhibition properties in ammonia-containing aqueous environments. The interaction mechanisms of these materials with copper surfaces were systematically investigated using electrochemical techniques and surface characterization methods. Based on these analyses, a comprehensive mechanistic model was developed to explain the interplay of the observed factors. The results demonstrated that the corrosion inhibition performance of zeolitic imidazolate frameworks (ZIFs), a representative class of MOFs, is significantly influenced by the surrounding environment. Specifically, experimental analysis revealed a competitive interaction between NH3 and the ZIF ligand in complex reactions, leading to structural instability of the ZIFs. This instability compromises the protective layer formed on the copper surface, resulting in a reduction of up to 60% in corrosion inhibition efficiency and, consequently, insufficient long-term durability.
Keywords: MOFs; ZIFs; ammonia-containing aqueous environments; copper; corrosion inhibition.
Copyright © 2025 Cao, Wu, Ge, Yang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Aboraia A. M., Darwish A. A. A., Polyakov V., Erofeeva E., Butova V., Zahran H. Y., et al. (2020). Structural characterization and optical properties of zeolitic imidazolate frameworks (ZIF-8) for solid-state electronics applications. Opt. Mater. 100, 109648. 10.1016/j.optmat.2019.109648 - DOI
-
- Abtan N. S., Al-Hamid M. A. I., Kadhim L. A., Sayyid F. F., Noori F. T. M., Kadum A., et al. (2024). Unlocking the power of 4-Acetamidoantipyrine: a promising corrosion inhibitor for preserving mild steel in harsh hydrochloric acid environments. Prog. Color, Colorants and Coatings 17. 10.30509/pccc.2023.167147.1223 - DOI
-
- Ali H. A., El-Hossiany A. A., Abousalem A. S., Ismail M. A., Fouda E. A. S., Ghaith E. A. (2024). Synthesis of new binary trimethoxyphenylfuran pyrimidinones as proficient and sustainable corrosion inhibitors for carbon steel in acidic medium: experimental, surface morphology analysis, and theoretical studies. BMC Chem. 18 (1), 182. 10.1186/s13065-024-01280-6 - DOI - PMC - PubMed
-
- Aliyari D., Mahdavian M., Ramezanzadeh B. (2023). Assessment of synthesis conditions on the corrosion inhibitive features of ZIF-67 MOF. Surf. Coatings Technol. 474, 130060. 10.1016/j.surfcoat.2023.130060 - DOI
-
- Bai W., Zhang X., He Z., Qian Y., Jian R., Lin Y., et al. (2023). Intelligent anti-corrosion coating with multiple protections using active nanocontainers of ZnAl LDH equipped with ZIF-8 encapsulated environment-friendly corrosion inhibitors. Prog. Org. Coatings 185, 107940. 10.1016/j.porgcoat.2023.107940 - DOI
LinkOut - more resources
Full Text Sources

