Reversed phase HPLC analysis of mobocertinib and its impurities and studies on the structure and biological activity of a new degradation product
- PMID: 41059051
- PMCID: PMC12497807
- DOI: 10.3389/fchem.2025.1659507
Reversed phase HPLC analysis of mobocertinib and its impurities and studies on the structure and biological activity of a new degradation product
Abstract
Background: Mobocertinib, an epidermal growth factor receptor tyrosine kinase inhibitor, is prescribed for the treatment of non-small cell lung cancer characterized by epidermal growth factor receptor exon 20 insertion mutations. The presence of impurities generated during its synthesis or storage may compromise the drug's efficacy and safety. Therefore, a comprehensive investigation of these impurities and the implementation of rigorous quality control measures are of paramount importance. However, robust analytical methods for the simultaneous and accurate detection of mobocertinib and its related impurities are currently lacking.
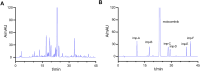
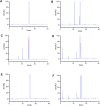
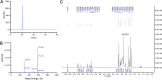
Methods: This study developed a novel reversed-phase high-performance liquid chromatography method (RP-HPLC) for separating and analysing mobocertinib and its impurities. An Agilent 5HC-C18 column (4.6 mm × 250 mm, 5 μm) was used to separate Mobocertinib and its related substances. The mobile phase composition, gradient elution program, and ultraviolet detection wavelength were optimized. Additionally, a new product (imp-A) was found during the forced degradation test. Its structure was elucidated by RP-HPLC, nuclear magnetic resonance (NMR) and high resolution mass spectrometry (HRMS). The biological activity of imp-A was preliminarily evaluated by methyl thiazolyl tetrazolium (MTT) assay.
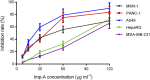
Results: The RP-HPLC method developed in this study was validated in accordance with ICH guidelines, demonstrating satisfactory specificity, precision, stability, repeatability, accuracy, and robustness. The method exhibited good linearity over the concentration range of 0.1-20 μg mL-1. The limits of detection and quantitation for mobocertinib were determined to be 0.02 μg mL-1 and 0.05 μg mL-1, respectively. The structure of imp-A was successfully characterized, and its formation mechanism was elucidated. Furthermore, imp-A was found to inhibit the growth of various tumor cell lines.
Conclusion: The developed RP-HPLC method is suitable for the simultaneous detection of mobocertinib and its impurities, providing significant advantages for process development and quality control. Imp-A, a novel compound, demonstrated promising anticancer activity in vitro. However, further in vivo studies are required to fully assess its therapeutic potential, which may hold promise for clinical applications in cancer treatment.
Keywords: RP-HPLC; anticancer effect; impurity; mobocertinib; novel product.
Copyright © 2025 Ni, Qin, Wu, Xu, Luo and Cai.
Conflict of interest statement
Author WW was employed by Sinopharm holding Nantong Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

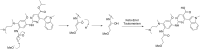
References
-
- Baksam V. K., Saritha N., Mohan S. K., Shandilya S., Kumar P. (2021). Identification and characterization of prothionamide degradation impurities by mass spectrometry, NMR spectroscopy, and ultra high performance liquid chromatography method development. J. Sep. Sci. 44, 2078–2088. 10.1002/jssc.202100050 - DOI - PubMed
-
- Chen H., Shah A., Kato S., Griffin R., Zhang S., Pusalkar S., et al. (2024). Metabolism and excretion of [14C]Mobocertinib, a selective covalent inhibitor of epidermal Growth factor receptor (EGFR) exon 20 insertion mutations, in healthy Male subjects. Drug Metab. Dispos. 52, 1115–1123. 10.1124/dmd.124.001841 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

