Effects of a 24-week multicomponent training program on functional capacity, persistent symptoms, body composition, and physical activity in patients significantly affected by COVID-19: the COVID-19 and REhabilitation study (CORE-study)-randomized clinical trial
- PMID: 41059218
- PMCID: PMC12497707
- DOI: 10.3389/fspor.2025.1549132
Effects of a 24-week multicomponent training program on functional capacity, persistent symptoms, body composition, and physical activity in patients significantly affected by COVID-19: the COVID-19 and REhabilitation study (CORE-study)-randomized clinical trial
Abstract
Background: COVID-19 can lead to reduced functional capacity, loss of muscle mass, and lasting and persistent symptoms, resulting in reduced physical activity.
Objective: To evaluate the effects of a multicomponent training on functional capacity, persistent symptoms, body composition, pulmonary function, and physical activity levels in patients significantly impaired by SARS-CoV-2.
Methods: The participants were randomly assigned (1:1) to either the intervention group (IG), which received multicomponent training (balance/aerobic/resistance), or the control group (CG). Functional capacity [6 min walk test (6MWT)-primary outcome, sit and reach, sit-to-stand, timed up and go], persistent symptoms (dyspnea, fatigue, post-COVID functional status, frailty), body composition (dual-energy x-ray absorptiometry and bioimpedance), pulmonary function, and physical activity levels (accelerometry) were evaluated at baseline and after 24 weeks. Generalized estimating equations were used, with the significance level set at α = 0.05. Outcomes were analyzed by intention-to-treat (ITT) and per-protocol (PP) approaches. Effect sizes were calculated from the mean difference between groups of changes between pre- and post-intervention.
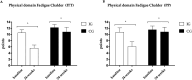
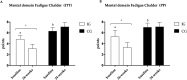
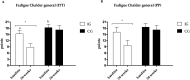
Results: Forty participants [age = 52.00 (12.93) years, 19 women] were included. The primary outcome 6MWT showed improvement in both groups in the ITT analysis (IG: 35.5 m, 95% CI: -3.0 to 74.1; CG: 37.4 m, 95% CI: -5.26 to 80.2) and in the IG (87.6 m, 95% CI: 50.6-124.4) in the PP analysis. The IG showed a reduction in mental fatigue (-1.7 points, 95% CI: -0.5 to 3.5) and general fatigue (-6.5 points, 95% CI: -9.4 to -3.5) in our ITT analysis. The IG also revealed improvement in timed up and go test (-1.6 s, 95% CI: -2.6 to -0.6), mental fatigue (-2.0points, 95% CI: -3.6 to 0.7), general fatigue (-6.4points, 95% CI: -11.0 to -1.6), and a protective effect against increased body fat in PP analysis.
Conclusion: This program was effective in improving fatigue in patients previously significantly affected by COVID-19.
Keywords: COVID-19; SARS-CoV-2; physical capacity; physical exercise; rehabilitation.
© 2025 Danielevicz, Diesel, dos Santos, Sirydakis, de Melo, Constantini, Hansen, Gerage, Freitas, Rech, Fonseca, Maurici and Delevatti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- World Health Organization (WHO). Clinical Management of COVID-19: Living Guideline. Geneva: World Health Organization; (2022). - PubMed
-
- Debeaumont D, Boujibar F, Ferrand-Devouge E, Artaud-Macari E, Tamion F, Gravier FE, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther. (2021) 101:pzab099. 10.1093/ptj/pzab099 - DOI - PMC - PubMed
-
- COVID-19 Rapid Guideline: Managing the Long-term Effects of COVID-19. London: National Institute for Health and Care Excellence (NICE) (2020). (NICE Guideline, N.188). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK567261/7 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

