The Cryptic Nature of Fe-S Clusters: A Case Study of the Hepatitis B HBx Oncoprotein
- PMID: 41059246
- PMCID: PMC12499515
- DOI: 10.3390/inorganics11120475
The Cryptic Nature of Fe-S Clusters: A Case Study of the Hepatitis B HBx Oncoprotein
Abstract
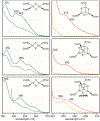
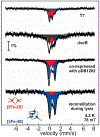
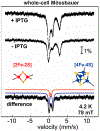
Fe-S clusters are ubiquitous inorganic cofactors found in proteins across all domains of life, including viruses. Their prevalence stems from their unique redox and structural plasticity that supports functions ranging from electron transfer and catalysis to stabilization of protein structure. Although the ability of Fe-S clusters to exchange electrons is often functionally crucial, it can also act as an Achilles heel when these cofactors are exposed to oxidizing conditions, often leading to their degradation. This O2 sensitivity has rendered certain Fe-S clusters untraceable, particularly when the nascent proteins are isolated under ambient conditions. As a consequence of this O2 sensitivity, a growing number of proteins with roles in viral infection have been found to harbor Fe-S clusters rather than the annotated Zn2+ cofactor. The enigmatic protein X (HBx) of the Hepatitis B Virus is a multifunctional protein essential for viral replication and development of liver disease. Although HBx has defied biochemical characterization for over forty years, it has been shown to coordinate a redox-active Fe-S cluster that represents a significant feature for establishing its molecular function. The present review narrates the approaches to validate the HBx metallocofactor that can be broadly applied as a guide for uncovering the presence of Fe-S clusters in proteins with non-canonical sequence motifs.
Keywords: EPR spectroscopy; Fe-S clusters; Hepatitis B; Mössbauer spectroscopy; UV/VIS.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures


References
-
- Beinert H; Holm RH; Münck E Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 1997, 277, 653–659. - PubMed
-
- Johnson DC; Dean DR; Smith AD; Johnson MK Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem 2005, 74, 247–281. - PubMed
-
- Fontecave M Iron-sulfur clusters: Ever-expanding roles. Nat. Chem. Biol 2006, 2, 171–174. - PubMed
-
- Honarmand Ebrahimi K; Ciofi-Baffoni S; Hagedoorn PL; Nicolet Y; Le Brun NE; Hagen WR; Armstrong FA Iron–sulfur clusters as inhibitors and catalysts of viral replication. Nat. Chem 2022, 14, 253–266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
