Efficacy and safety of biliary stenting combined with 125I seed implantation for the treatment of advanced extrahepatic cholangiocarcinoma
- PMID: 41059263
- PMCID: PMC12497782
- DOI: 10.3389/fsurg.2025.1608312
Efficacy and safety of biliary stenting combined with 125I seed implantation for the treatment of advanced extrahepatic cholangiocarcinoma
Abstract
Background: The implantation of 125I seed is expected to improve the prognosis of patients undergoing stent placement for advanced extrahepatic cholangiocarcinoma (eCCA), but its efficacy and safety remain unclear.
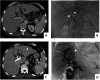
Methods: Forty advanced eCCA patients who received percutaneous transhepatic biliary stenting (PTBS) (control group) and 40 PTBS combined with 125I seed implantation (125I group) were retrospectively analyzed. Changes in serum biochemical indicators and tumor markers as well as the occurrence of complications were observed in the two groups, and the durations of stent patency and survival were compared.
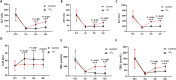
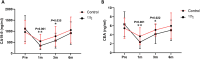
Results: The general information and preoperative baseline data did not significantly differ between the two groups (P < 0.05). Regardless of whether PTBS was combined with 125I seed implantation, the ALT/AST levels of patients after operation were significantly lower, jaundice was relieved. And the improvements in postoperative liver function and jaundice in patients in 125I group were better than those in control group. In addition, tumor markers in the two groups decreased significantly, and the decrease was more significant in patients in 125I group. There was no significant difference in the total complication rate between the two groups. The stent patency time and overall survival of the patients in the 125I group were longer than those in control group.
Conclusion: Biliary stenting combined with 125I seed implantation is a safe and effective treatment for patients with advanced eCCA, and it is superior to biliary stenting alone in improving liver function and prolonging the duration of stent patency and survival time.
Keywords: 125I seed implantation; biliary stenting; extrahepatic cholangiocarcinoma; postoperative survival; stent patency time.
© 2025 Cheng, Wang, Miao and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

