Use of isavuconazole in critically ill patients in intensive care units: a prospective, observational, multicentre, cohort study
- PMID: 41059267
- PMCID: PMC12498522
- DOI: 10.1093/jacamr/dlaf177
Use of isavuconazole in critically ill patients in intensive care units: a prospective, observational, multicentre, cohort study
Abstract
Objectives: In this multicentre, prospective study, we aimed to describe the use of isavuconazole in critically ill adult patients in ICU, in terms of patient characteristics, infection characteristics and outcomes.
Methods: Prospective, observational study of ICU patients treated with isavuconazole from January 2023 to 30 April 2025 in 17 centres (ISA-SITA study within the MULTI-SITA project).
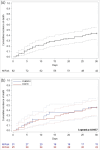
Results: A total of 177 ICU patients treated with isavuconazole were included in the study. Most patients showed at least one European Organisation for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) or FUNgal Diseases in adult patients in Intensive Care Unit (FUNDICU) host factor (141/177, 79.7%). Overall, 82/177 patients (46.3%) had either proven or probable invasive mould disease (6 and 76, respectively, mostly invasive pulmonary aspergillosis). In patients with proven or probable disease, 30-day mortality was 44.0%, and 90-day mortality was 62.2%. In multivariable analyses, SOFA score (HR 1.14 per one point increase, 95% CI 1.03-1.26, P = 0.010) and concomitant bacterial pneumonia (HR 2.32, 95% CI 1.17-4.59, P = 0.016) were associated with 30-day mortality, whereas prior hospitalization (HR 2.26, 95% CI 1.19-4.27, P = 0.013) and SOFA score (HR 1.17 per one point increase, 95% CI 1.07-1.28, P < 0.001) were associated with 90-day mortality.
Conclusions: Diverse patterns of isavuconazole use were observed in a large cohort of critically ill adult patients, and the drug was well tolerated. Mortality was lower than many previous estimates in critically ill patients and could serve as a basis for future standardized comparisons.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- Maertens JA, Raad II, Marr KA et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387: 760–9. 10.1016/S0140-6736(15)01159-9 - DOI - PubMed
-
- Cornely OA, Alastruey-Izquierdo A, Arenz D et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019; 19: e405–21. 10.1016/S1473-3099(19)30312-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
