Metabolite to Modifier: Lactate and Lactylation in the Evolution of Tumors
- PMID: 41059490
- PMCID: PMC12497687
- DOI: 10.1002/mco2.70413
Metabolite to Modifier: Lactate and Lactylation in the Evolution of Tumors
Abstract
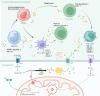
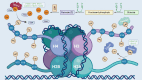
Lactate, once dismissed as a mere by-product of cancer metabolism, has emerged as a pivotal factor in tumor progression, exerting diverse effects on metabolic reprogramming and immune modulation. Lactate enhances tumor cell adaptability through sustained glycolysis and concurrently shapes the tumor microenvironment by modulating immune, stromal, and endothelial cell function. This review highlights the evolving understanding of lactate's role, extending beyond the Warburg effect to its regulatory capacity via lactylation, a recently identified post-translational modification. The complex interaction between lactate and tumor biology is examined, emphasizing its influence on the tumor microenvironment and immune dynamics. Additionally, potential therapeutic strategies targeting lactate metabolism and transport are explored, along with lactylation regulation by histone-modifying enzymes. Inhibitors targeting lactate production and transport, especially those against lactate dehydrogenase (LDH) and monocarboxylate transporters (MCTs), have shown considerable potential in preclinical and early clinical studies. Recent advancements are discussed, underscoring the potential of integrating metabolic regulation with immunotherapies, thereby offering a dual pathway in cancer treatment. These insights establish lactate and lactylation as pivotal modulators of tumor biology and highlight their potential as targets in precision oncology.
Keywords: cancer therapy; immune modulation; lactate; lactylation; metabolic reprogramming; tumor microenvironment.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Ferguson B. S., Rogatzki M. J., Goodwin M. L., Kane D. A., Rightmire Z., and Gladden L. B., “Lactate Metabolism: Historical Context, Prior Misinterpretations, and Current Understanding,” European Journal of Applied Physiology 118, no. 4 (2018): 691–728. - PubMed
-
- Zhao Y., Li M., Yao X., et al., “HCAR1/MCT1 Regulates Tumor Ferroptosis Through the Lactate‐Mediated AMPK‐SCD1 Activity and Its Therapeutic Implications,” Cell Reports 33, no. 10 (2020): 108487. - PubMed
-
- Brown T. P. and Ganapathy V., “Lactate/GPR81 Signaling and Proton Motive Force in Cancer: Role in Angiogenesis, Immune Escape, Nutrition, and Warburg Phenomenon,” Pharmacology & Therapeutics 206 (2020): 107451. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
