In vitro activity of cefiderocol against nosocomial Acinetobacter baumannii
- PMID: 41059689
- PMCID: PMC12584741
- DOI: 10.1128/spectrum.00844-25
In vitro activity of cefiderocol against nosocomial Acinetobacter baumannii
Abstract
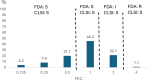
The emergence and spread of third-/fourth-generation cephalosporin and/or carbapenem-resistant Acinetobacter baumannii have become a significant global public health concern, making new treatment alternatives necessary. Thus, the present study aimed to assess in vitro cefiderocol activity against clinical isolates of A. baumannii and analyze their relationship with extended-spectrum β-lactamases (ESBLs) and carbapenemases. Ninety-five A. baumannii clinical isolates were included in the study. Susceptibility to 12 antimicrobial agents was established by automated methods and/or disk diffusion, while that of colistin was determined following microdilution and that of cefiderocol by microdilution using iron-depleted broth. The presence of blaCTX-M, blaPER, blaVEB, blaGES, blaVIM, blaIMP, blaIMI, blaKPC, blaNDM, blaOXA-23G, blaOXA-24G, blaOXA-48G, and blaOXA-58G was established by PCR. The results showed extremely high levels of resistance (>80%) to all the tested antibacterial agents except colistin (11.6%) and cefiderocol (Clinical and Laboratory Standards Institute [CLSI]: 0%; US Food and Drug Administration [FDA]: 1.1%). Following FDA criteria, 22.1% of isolates were intermediate to cefiderocol, with 68.4% of isolates surpassing the European Committee on Antimicrobial Susceptibility Testing epidemiological cut off. Seven colistin-resistant isolates were only susceptible to cefiderocol following CLSI breakpoints, four of them qualifying as cefiderocol-intermediate following FDA breakpoints. No association between the presence of ESBLs or carbapenemases and cefiderocol minimum inhibitory concentration levels was observed. The present results show the potential utility of cefiderocol in the treatment of A. baumannii infections, highlighting the need for judicious use and continuous surveillance to prevent the emergence of cefiderocol-resistant A. baumannii clones.IMPORTANCEAntibiotic resistance is a silent pandemic challenging the treatment of infectious diseases worldwide, but also other medical practices, as, for instance, organ transplantation procedures. In Peru, current levels of antimicrobial resistance are worrisome. In this scenario, we have determined the in vitro activity of cefiderocol against a series of Acinetobacter baumannii exhibiting high levels of resistance to commonly used antibiotics. This activity is independent of the presence of the most common extended-spectrum beta-lactamases or carbapenemases. Obtained results showed the potential of cefiderocol to become an alternative for the treatment of this type of microorganism, but the high number of isolates bordering the considered breakpoint, despite the lack of use of cefiderocol in the country, also shows the need for a prudent use of this antibiotic to maximize its utility while minimizing the selection of resistant isolates.
Keywords: OXA-24; antibiotic resistance; beta-lactamases; carbapenemases; cefiderocol; middle-income countries.
Conflict of interest statement
J.R. and M.J.P. have research grants from Shionogi and Pfizer.
Figures
References
-
- Ventura M, Oporto-Llerena R, Espinoza K, Guibert F, Quispe AM, Vilar N, López M, Rojo-Bezares B, Sáenz Y, Ruiz J, J Pons M. 2024. Antimicrobial resistance and associated risk factors in Escherichia coli isolated from Peruvian dogs: a focus on extended-spectrum β-lactamases and colistin. Vet World 17:880–887. doi: 10.14202/vetworld.2024.880-887 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous