Hidden Splicing Variants in Inherited Retinal Degeneration: Discovery and Functional Insight
- PMID: 41060150
- PMCID: PMC12517372
- DOI: 10.1167/iovs.66.13.12
Hidden Splicing Variants in Inherited Retinal Degeneration: Discovery and Functional Insight
Abstract
Purpose: To enhance the molecular diagnosis of inherited retinal degeneration (IRD) by systematically identifying pathogenic splicing variants and characterizing their transcript-level consequences.
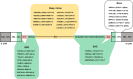
Methods: We analyzed 738 IRD families who underwent targeted gene panel sequencing. A splicing variant detection pipeline, integrating two computational algorithms (SpliceAI and dbscSNV_ADA) with functional validation via minigene assays, was implemented to detect splice-disrupting variants beyond canonical sites.
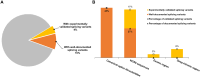
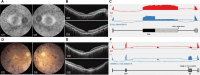
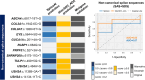
Results: Splicing variants accounted for 14% of genetically diagnosed families. Of these, 4% were newly identified through our combined computational and experimental platform. Notably, 28% of all splice-disrupting variants, located in noncanonical, exonic, or deep-intronic regions, would likely have been missed by conventional analysis pipelines, which typically prioritize protein-coding changes and canonical splice sites, and often lack systematic evaluation of splicing effects beyond these regions. Five recurrent splice-disrupting variants were observed across multiple families, including EYS:c.5644+5G>A, which caused exon truncation and was found in 11 unrelated families. Functional assays confirmed aberrant splicing, and the associated phenotypes were consistent with known disease presentations.
Conclusions: This study demonstrates the utility of a combined splicing variant detection platform in uncovering hidden pathogenic variants and improving IRD diagnostic yield. These findings have implications for refining genetic testing and guiding the development of splicing-targeted therapies.
Conflict of interest statement
Disclosure:
Figures

References
-
- RetNet: disease table, https://web.sph.uth.edu/RetNet/disease.htm. Accessed March 29, 2023.
-
- Sundaresan Y, Banin E, Sharon D. Exonic variants that affect splicing—an opportunity for “hidden” mutations causing inherited retinal diseases. In: Ash JD, Pierce E, Anderson RE, Bowes Rickman C, Hollyfield JG, Grimm C, eds. Retinal Degenerative Diseases XIX. Cham. Switzerland: Springer International Publishing; 2023: 183–187. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

