Comparative Clinical Outcomes of Nusinersen and Gene Therapy in Spinal Muscular Atrophy Type 1
- PMID: 41060652
- PMCID: PMC12508997
- DOI: 10.1001/jamanetworkopen.2025.36348
Comparative Clinical Outcomes of Nusinersen and Gene Therapy in Spinal Muscular Atrophy Type 1
Abstract
Importance: Therapeutic advances have transformed the prognosis of spinal muscular atrophy (SMA). Given the lifelong implications of these innovative therapies, comparative data on their efficacy are urgently required.
Objective: To compare clinical outcomes of nusinersen and onasemnogene abeparvovec gene therapy as first-line treatments in children with SMA type 1 (SMA1).
Design, setting, and participants: This comparative effectiveness study used data from the French National SMA Registry from September 2016 to July 2024. The follow-up period started at treatment initiation and continued until July 22, 2024, or death. Children with genetically confirmed SMA1 (types a, b, or c) treated within 6 months of diagnosis with either nusinersen or gene therapy as first-line therapy and followed up for at least 24 months were included. Matching criteria included age, baseline score on the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders, and respiratory and nutritional status at treatment initiation.
Exposure: First-line treatment with either nusinersen or gene therapy.
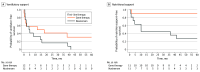
Main outcomes and measures: Outcomes included respiratory and nutritional support needs, motor function, and unsatisfactory clinical response (UCR)- a composite of death, treatment switch (or, for gene therapy, addition) due to inadequate response, initiation of feeding support, and/or failure to achieve independent sitting.
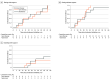
Results: Among 1366 patients enrolled in the registry, 309 were diagnosed with SMA1. Twenty-four children in 12 matched pairs met inclusion criteria (14 [58%] male; mean [SD] age at treatment initiation, 6.1 [3.0] months [range, 2.3-11.9 months]). Three patients (1 receiving gene therapy [8%], 2 receiving nusinersen [17%]) died within the first year of treatment. At 2 years posttreatment, 1 of the 11 surviving patients treated with gene therapy (9%) required nutritional support vs 5 of 10 (50%) treated with nusinersen, and nocturnal ventilation was required in 5 of 11 (45%) receiving gene therapy vs 8 of 10 (80%) receiving nusinersen. Motor outcomes were comparable between groups (mean [SE] intrapair difference in CHOP-INTEND score evolution, -1.69 [1.24] points; P = .17). UCR occurred in 8 of 12 patients (67%) receiving nusinersen and 3 of 12 (25%) receiving gene therapy.
Conclusions and relevance: In this comparative effectiveness study of children with SMA1, gene therapy was associated with lower incidence of UCR and fewer supportive care needs vs nusinersen. These exploratory findings warrant confirmation in larger studies.
Conflict of interest statement
Figures

References
-
- Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284-293. doi: 10.1016/S1474-4422(21)00001-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

