B7-H3-mediated reversal of CAR-T cell exhaustion induces a notable antitumour response in ovarian cancer models
- PMID: 41061481
- PMCID: PMC12539249
- DOI: 10.1016/j.ebiom.2025.105949
B7-H3-mediated reversal of CAR-T cell exhaustion induces a notable antitumour response in ovarian cancer models
Abstract
Background: Functional CAR-T cell exhaustion in the immunosuppressive tumour microenvironment remains the main barrier to the success of CAR-T cell therapy for treating solid tumours. Mesothelin (MSLN) has emerged as an attractive target for CAR-T cell therapy for several solid malignancies, including ovarian cancer. In this study, we aimed to investigate the role and mechanism of lipid metabolites in anti-MSLN CAR-T cell exhaustion in ovarian cancer cells.
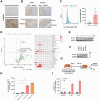
Methods: We engineered anti-MSLN CAR-T cells targeting ovarian cancer cells with high MSLN expression as a pivotal tool for in vitro and in vivo experiments. Moreover, liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed the critical role of oxylipin 12-HETE in the exhaustion of CAR-T cells. By employing structure-based high-throughput virtual screening (HTVS), we identified the inhibitor targeting B7-H3.
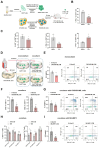
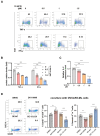
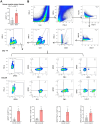
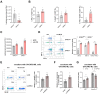
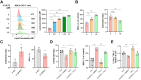
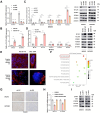
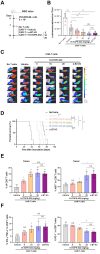
Findings: We demonstrated that GPR31-dependent 12-HETE accumulation in the ovarian cancer microenvironment drives CAR-T cell exhaustion via lipid peroxidation, impairing their antitumour efficacy. Genetic or pharmacological inhibition of the 12-HETE/GPR31 axis restored CAR-T cell cytotoxicity and proliferation, leading to significant tumour regression in murine models. Silencing B7-H3 relieved repression of FOXO3, leading to reduced 12-LOX expression and lower 12-HETE levels, which places B7-H3 upstream of this metabolic checkpoint. Through structure-based screening, we identified HI-TOPK-032 as a potent B7-H3 inhibitor that synergised with CAR-T cell therapy by reversing exhaustion markers (e.g., PD-1, TIM-3) and enhancing cytokine polyfunctionality. Combined HI-TOPK-032 and anti-PD-1 treatment achieved superior tumour control compared to monotherapies, particularly in B7-H3/12-LOX-high patient-derived xenografts, underscoring its precision therapeutic potential.
Interpretation: CAR-T cell therapy combined with HI-TOPK-032 is a promising novel strategy for treating MSLN-expressing solid tumours.
Funding: This study was funded by the National Natural Science Foundation of China (Grant number: 82503173), Beijing Hospitals Authority's Ascent Plan (Grant number: DFL20221201), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (Grant number: ZYLX202120), Beijing Natural Science Foundation (Grant number: 7162063), Capital Medical University Laboratory for Clinical Medicine and Gynecological Tumour Precise Diagnosis and Treatment Innovation Studio.
Keywords: 12-HETE; Anti-PD-1; B7-H3; CAR-T cells; CD8 T cell; HI-TOPK-032; Ovarian cancer.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no competing interests.
Figures


References
-
- Sadelain M. CD19 CAR T cells. Cell. 2017;171(7):1471. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

