Egg intake and cognitive function in healthy adults: A systematic review of the literature
- PMID: 41061594
- PMCID: PMC12538690
- DOI: 10.1016/j.jnha.2025.100696
Egg intake and cognitive function in healthy adults: A systematic review of the literature
Abstract
Background: Cognitive decline is a growing public health concern, particularly in aging populations. Eggs are a widely consumed, nutrient-dense food containing choline, phospholipids, tryptophan, and omega-3 fatty acids, which individually support cognitive processes such as memory, attention, and neurogenesis. While these individual nutrients have demonstrated benefits in supplementation studies, the cognitive effects of whole egg consumption are not well established. This systematic review aimed to evaluate the association between whole egg consumption and cognitive function in healthy adults.
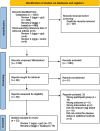
Methods: A systematic search of five electronic databases (Medline, Embase, CINAHL Plus, SCOPUS, and PsychInfo) was conducted from database inception through February 2025. Studies were included if they investigated whole egg intake in relation to cognitive outcomes in healthy adults. Risk of bias was assessed using tools appropriate to study design. Due to heterogeneity in study methods, outcomes were synthesised narratively. Cognitive outcomes were categorised into domains including global cognitive function, memory, executive function, language, processing speed, and dementia risk.
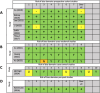
Results: Eleven studies met the inclusion criteria: one pre-post intervention study, six prospective cohort studies, three cross-sectional studies, and one case-control study. Study populations were predominantly older adults and included >38,000 participants. Two studies reported a reduced risk of dementia or cognitive impairment associated with moderate egg consumption (approximately 0.5-1 egg per day), while one study found increased risk at high intake levels (Over 1 egg per day). Several studies showed improvements in memory, verbal fluency, or processing speed with moderate-but not high-egg intake. The pre-post study reported improved reaction time following eight weeks of daily egg consumption (2 eggs per day). Heterogeneity in exposure measurement and cognitive testing methods limited direct comparisons across studies.
Discussion: Moderate whole egg consumption may be associated with improvements in cognitive outcomes in healthy adults, including reduced dementia risk and better memory performance. However, findings are inconsistent and limited by differences in study design, dietary assessment, and cognitive testing. Further well-controlled intervention studies are needed to determine optimal intake levels, explore mechanisms, and assess whether eggs can be integrated meaningfully into dietary strategies to support cognitive aging. (PROSPERO registration: 408532).
Keywords: Aging; Cognition; Dementia; Eggs; Nutrition; Systematic review.
Copyright © 2025 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Nil.
Figures
References
-
- McDonald W.M.M.D. Defining neurocognitive disorders. Am J Geriatr Psychiatry. 2011;19:909–914. - PubMed
-
- Cheng G., Huang C., Deng H., Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. - PubMed
-
- Comijs H.C., Kriegsman D.M.W., Dik M.G., Deeg D.J.H., Jonker C., Stalman W.A.B. Somatic chronic diseases and 6-year change in cognitive functioning among older persons. Arch Gerontol Geriatr. 2009;48:191–196. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

