Neural stem cell-delivered oncolytic virus via intracerebroventricular administration enhances glioblastoma therapy and immune modulation
- PMID: 41062177
- PMCID: PMC12516981
- DOI: 10.1136/jitc-2025-012934
Neural stem cell-delivered oncolytic virus via intracerebroventricular administration enhances glioblastoma therapy and immune modulation
Abstract
Background: Glioblastoma (GBM) is a highly aggressive brain tumor with poor prognosis and limited treatment options. Oncolytic virus (OV) therapy holds promise but is hindered by immune neutralization and poor tumor infiltration. Neural stem cells (NSCs) can enhance OV delivery, and intracerebroventricular (ICV) administration offers broader tumor access. This study evaluates NSC-OV therapy via ICV injection for improved tumor targeting and immune modulation in GBM.
Methods: NSCs were infected with OV and assessed for viral uptake and replication. In vitro assays examined NSC-OV effects on glioma proliferation and migration. In vivo xenograft and orthotopic models evaluated tumor targeting, therapeutic efficacy, and immune modulation. Humanized immune system mouse models enabled single-cell RNA sequencing and flow cytometry analysis of immune responses.
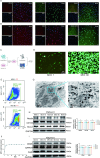
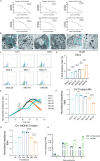
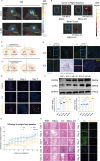
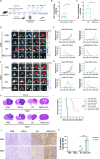
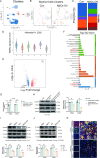
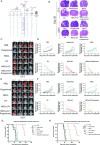
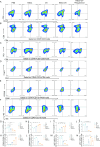
Results: NSCs retained their stemness after OV infection. NSCs-OV significantly inhibited glioma cell migration, proliferation, and colony formation in vitro. In orthotopic GBM models, NSCs-OV exhibited enhanced tumor homing, prolonged viral persistence, and reduced tumor burden while minimizing inflammation and systemic toxicity. NSCs protected OV from neutralizing antibodies, leading to sustained efficacy. Single-cell RNA sequencing indicated that NSCs-OV therapy reduced tumor-promoting inflammation by downregulating S100A8/A9, markers of myeloid-derived suppressor cells (MDSCs) and chemotactic factors that recruited MDSCs into tumors. Combining NSCs-OV with Paquinimod further suppressed tumor growth by reducing MDSCs and increasing activated T cells.
Conclusions: NSCs serve as efficient OV carriers, enhancing tumor targeting, suppressing GBM progression, and modulating the immune landscape. The combination with Paquinimod amplifies therapeutic benefits, offering a promising strategy for improving GBM treatment outcomes.
Keywords: Central Nervous System Cancer; Myeloid-derived suppressor cell - MDSC; Oncolytic virus; Stem cell; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: KZ held ownership interests and owned patents for YSCH-01 at Yuansong Biotechnology. The other authors have no potential conflicts of interest to disclose.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
