Temporal and context-dependent requirements for the transcription factor Foxp3 expression in regulatory T cells
- PMID: 41062655
- PMCID: PMC12571910
- DOI: 10.1038/s41590-025-02295-4
Temporal and context-dependent requirements for the transcription factor Foxp3 expression in regulatory T cells
Abstract
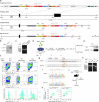
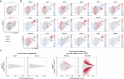
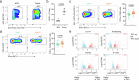
Regulatory T (Treg) cells, expressing the transcription factor Foxp3, are obligatory gatekeepers of immune responsiveness, yet the mechanisms by which Foxp3 governs the Treg transcriptional network remain incompletely understood. Using a novel chemogenetic system of inducible Foxp3 protein degradation in vivo, we found that while Foxp3 was indispensable for the establishment of transcriptional and functional programs of newly generated Treg cells, Foxp3 loss in mature Treg cells resulted in minimal functional and transcriptional changes under steady state. This resilience of the Foxp3-dependent program in mature Treg cells was acquired over an unexpectedly long timescale; however, in settings of severe inflammation, Foxp3 loss led to a pronounced perturbation of Treg cell transcriptome and fitness. Furthermore, tumoral Treg cells were uniquely sensitive to Foxp3 degradation, which led to impairment in their suppressive function and tumor shrinkage in the absence of pronounced adverse effects. These studies demonstrate a context-dependent differential requirement for Foxp3 for Treg transcriptional and functional programs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.Y.R. is a Scientific Advisory Board member and has equity in Sonoma Biotherapeutics, RAPT Therapeutics, Coherus BioSciences, Santa Ana Bio, Odyssey Therapeutics, Nilo Therapeutics and Vedanta Biosciences; he is also a Scientific Advisory Board member of BioInvent and Amgen and a co-inventor of a CCR8+ Treg cell depletion IP licensed to Takeda, which is unrelated to the content of this publication. Z.-M.W. is an employee of Genentech, which is unrelated to the content of this publication. The other authors declare no competing interests.
Figures














Update of
-
Temporal and Context-Dependent Requirements for the Transcription Factor Foxp3 Expression in Regulatory T Cells.Res Sq [Preprint]. 2025 May 14:rs.3.rs-6596747. doi: 10.21203/rs.3.rs-6596747/v1. Res Sq. 2025. Update in: Nat Immunol. 2025 Nov;26(11):2059-2073. doi: 10.1038/s41590-025-02295-4. PMID: 40470210 Free PMC article. Updated. Preprint.
References
-
- Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol.4, 330–336 (2003). - PubMed
-
- Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science299, 1057–1061 (2003). - PubMed
-
- Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol.4, 337–342 (2003). - PubMed
-
- Gavin, M. A. et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature445, 771–775 (2007). - PubMed
-
- Lin, W. et al. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol.8, 359–368 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- CA008748/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 CA008748/CA/NCI NIH HHS/United States
- R37 AI034206/AI/NIAID NIH HHS/United States
- R01 AI034206/AI/NIAID NIH HHS/United States
- T32 GM152349/GM/NIGMS NIH HHS/United States
- AI034206/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- DP2AI171161/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- DP2 AI171161/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

