Elevated VAMP8 expression promotes cervical cancer progression by enhancing autophagy via HIF-1 pathway
- PMID: 41063209
- PMCID: PMC12505561
- DOI: 10.1186/s12916-025-04378-3
Elevated VAMP8 expression promotes cervical cancer progression by enhancing autophagy via HIF-1 pathway
Abstract
Background: Cervical cancer, prevalent in low- and middle-income countries, is primarily caused by high-risk HPV16. Vesicle-Associated Membrane Protein 8 (VAMP8), involved in vesicle trafficking and autophagy, may influence HPV16-related cervical cancer progression.
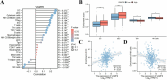
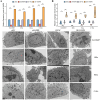
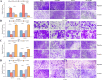
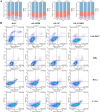
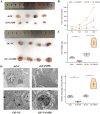
Methods: VAMP8 expression was evaluated in cervical tissue specimens from patients with HPV16-positive lesions (including low- and high-grade squamous intraepithelial lesions and cancer) and HPV-negative normal controls using proteomics, qPCR, and immunohistochemistry. A Cox proportional hazards model for prognosis was developed using immunohistochemical data from a cohort of cervical cancer patients. The clinical significance of VAMP8 was further assessed using RNA-seq and clinical data from The Cancer Genome Atlas-Cervical Cancer (TCGA-CESC) cohort. The effects of VAMP8 on autophagy and tumor progression were examined in HPV16 E6/E7-immortalized cervical epithelial cells (Ect1/E6E7) and cervical cancer cell lines (SiHa, HeLa, C-33A) in vitro, and in a SiHa xenograft model in vivo. Transcriptomic analysis of Ect1/E6E7 and SiHa cells identified VAMP8-regulated pathways. Chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assays in SiHa cells were used to confirm the regulation of the HIF-1 pathway.
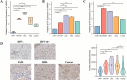
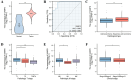
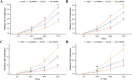
Results: VAMP8 was upregulated in HPV16-positive samples, particularly in low-grade squamous intraepithelial lesions (LSIL). Elevated VAMP8 correlated with poor survival outcomes and advanced tumor stages. VAMP8 enhanced autophagy and reduced proliferation and invasiveness in HPV16-positive cervical cells but increased in established cancer cell lines. In vivo, VAMP8 overexpression promoted tumor growth and autophagy. The HIF-1 pathway emerged as a key regulatory axis of VAMP8, enhancing hypoxic responses and angiogenesis.
Conclusion: Elevated VAMP8 in HPV16-associated cervical cancer promotes tumor progression by enhancing autophagy via the HIF-1 pathway, suggesting its potential as a diagnostic and prognostic biomarker.
Keywords: Autophagy; Biomarker; Cervical cancer; HIF-1 pathway; HPV16; VAMP8.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in full accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Obstetrics and Gynecology Hospital of Fudan University for the use of human clinical specimens (Approval No. 2019–53). All participants provided written informed consent prior to their inclusion in the study. All animal experiments were performed in compliance with institutional guidelines and were reviewed and approved by the Animal Care and Use Committee of the Obstetrics and Gynecology Hospital of Fudan University. The study is reported in accordance with the ARRIVE guidelines for animal experiments and the TRIPOD statement for the prognostic prediction study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

