Utility of metastasis-directed radiotherapy with and without hormonal therapy in management of oligometastatic prostate cancer
- PMID: 41063390
- PMCID: PMC12582589
- DOI: 10.1093/jncics/pkaf096
Utility of metastasis-directed radiotherapy with and without hormonal therapy in management of oligometastatic prostate cancer
Abstract
Background: Metastasis-directed radiotherapy (MDT) is the mainstay in management of oligometastatic prostate cancer (PCa), and PSMA-PET is currently the most sensitive imaging modality for localizing PCa metastases. The efficacy of MDT guided by PSMA-PET imaging with and without androgen deprivation therapy (ADT) ± androgen-receptor pathway inhibitor (ARPI) has not yet been well characterized. We sought to evaluate the efficacy of PSMA PET-guided MDT.
Methods: This is a single-institutional retrospective study of patients diagnosed with metastatic PCa by PSMA-PET imaging who were treated with MDT. Survival analyses were performed using the Kaplan-Meier method with Cox proportional hazards testing for significance. Cumulative incidence analyses were performed with Gray's testing for significance.
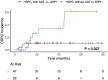
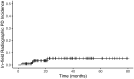
Results: One hundred and ninety-four metastatic lesions from 101 patients identified by PSMA PET were irradiated with MDT. Forty-seven of the 79 (59%) patients with hormone-sensitive PCa (HSPC) received ADT ± ARPI along with MDT. Four of 194 lesions (2.1%) demonstrated radiographic progression after MDT, with a median follow-up of 22.4 months. Two-year cumulative incidence of progression from HSPC to CRPC was 11% in patients who received ADT ± ARPI and 35% in those who did not (P = .027). Median biochemical progression free survival of patients with CRPC, HSPC treated without ADT or ARPI, and HSPC treated with ADT ± ARPI was 5.4, 7.6, and 43.9 months, respectively (P < .0001). No Grade 3-5 adverse effects were observed.
Conclusions: MDT guided by PSMA-PET imaging is well-tolerated and delays biochemical progression in patients with CRPC and HSPC, with a greater effect observed in patients also receiving ADT ± ARPI.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
J.C.H., who is a JNCI Cancer Spectrum Associate Editor and co-author on this paper, was not involved in the editorial review or decision to publish the manuscript.
Figures

References
-
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10. - PubMed
-
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18-e28. - PubMed
-
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446-453. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Medical
Miscellaneous
