Cuproptosis Facilitates Chronic Skin Inflammation by Regulating the α-Ketoglutarate/H3K4me3/Ferritin Heavy Chain 1 Signaling Pathway-Mediated Ferroptosis
- PMID: 41064128
- PMCID: PMC12501494
- DOI: 10.1002/mco2.70425
Cuproptosis Facilitates Chronic Skin Inflammation by Regulating the α-Ketoglutarate/H3K4me3/Ferritin Heavy Chain 1 Signaling Pathway-Mediated Ferroptosis
Abstract
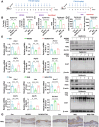
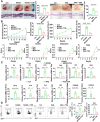
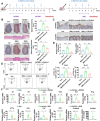
Dysregulated copper homeostasis is implicated in inflammatory skin diseases such as psoriasis and atopic dermatitis (AD), but the role of cuproptosis remains poorly defined. This study aimed to elucidate the role and mechanism of cuproptosis in inflammatory skin diseases. Transcriptome analysis of patient lesions revealed significant alterations in cuproptosis-related genes correlating with disease-specific pathological features. These cuproptosis-related gene expression signatures demonstrated strong clinical relevance to therapeutic efficacy in both psoriasis and AD cohorts. Functional validation using disease models showed that pharmacologically inhibiting cuproptosis with the copper chelator tetrathiomolybdate (TTM), or genetically knocking down the copper importer SLC31A1, effectively alleviated chronic skin inflammation and hallmark pathological changes induced by imiquimod (IMQ) or calcipotriol (MC903). Mechanistically, we uncovered that SLC31A1-mediated cuproptosis promotes intracellular α-ketoglutarate (α-KG) accumulation, driving activation of the lysine demethylase KDM5B. Activated KDM5B specifically demethylates H3K4me3 marks at the promoter of the ferroptosis regulator ferritin heavy chain 1 (FTH1), suppressing its transcription and consequently sensitizing keratinocytes to ferroptotic cell death, thereby amplifying inflammatory tissue damage. Our findings establish a fundamental pathogenic SLC31A1/KDM5B/FTH1 molecular axis linking dysregulated copper metabolism and cuproptosis to ferroptosis execution in psoriasis and AD, providing significant mechanistic insights and pinpointing promising therapeutic targets for these refractory skin disorders.
Keywords: cuproptosis; ferroptosis; fth1; h3k4me3; inflammatory skin diseases; slc31a1.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Shiva S., Wang X., Ringwood L. A., et al., “Ceruloplasmin Is a NO Oxidase and Nitrite Synthase That Determines Endocrine NO Homeostasis,” Nature Chemical Biology 2, no. 9 (2006): 486–493. - PubMed
-
- Linder M. C. and Hazegh‐Azam M., “Copper Biochemistry and Molecular Biology,” American Journal of Clinical Nutrition 63, no. 5 (1996): 797S–811S. - PubMed
-
- Kim B.‐E., Nevitt T., and Thiele D. J., “Mechanisms for Copper Acquisition, Distribution and Regulation,” Nature Chemical Biology 4, no. 3 (2008): 176–185. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
