Acute Myocardial Infarction: Molecular Pathogenesis, Diagnosis, and Clinical Management
- PMID: 41064129
- PMCID: PMC12501504
- DOI: 10.1002/mco2.70418
Acute Myocardial Infarction: Molecular Pathogenesis, Diagnosis, and Clinical Management
Abstract
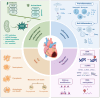
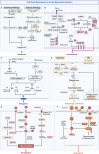
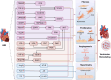
Acute myocardial infarction (AMI) is a cardiovascular disease characterized by myocardial necrosis resulting from acute coronary artery occlusion. Although standardized diagnostic and therapeutic protocols have markedly reduced its mortality, AMI remains a leading cause of death and disability worldwide. Contemporary AMI research has evolved from an initial focus on local myocardial injury to a broader perspective encompassing the entire disease course, including tissue damage, remodeling, and multi-organ interactions. This review systematically delineates the key molecular mechanisms underlying AMI and subsequent ventricular remodeling, while also exploring the complex interplay between the heart and other organs such as the gut, brain, kidney, and liver. From a clinical standpoint, we summarize the historical evolution of AMI diagnostic criteria and management strategies, highlighting current classification systems, novel diagnostic technologies, and the integration of artificial intelligence tools. Furthermore, we present recent evidence-based advances in established therapeutic approaches, along with emerging strategies ranging from cellular to genetic interventions. Future directions aim to integrate mechanistic insights with interdisciplinary clinical strategies to establish a systematic and precision-based framework for AMI prevention and management.
Keywords: acute myocardial infarction; inter‐organ crosstalk; myocardial ischemia/reperfusion infarction; pathogenesis; ventricular remodeling.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Zuin M., Rigatelli G., Temporelli P., et al., “Trends in Acute Myocardial Infarction Mortality in the European Union, 2012–2020,” European Journal of Preventive Cardiology 30, no. 16 (2023): 1758–1771. - PubMed
-
- Bugger H. and Pfeil K., “Mitochondrial ROS in Myocardial Ischemia Reperfusion and Remodeling,” Biochimica Et Biophysica Acta Molecular Basis of Disease 1866, no. 7 (2020): 165768. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
