A streamlined synthetic approach to the truncated linear trisaccharide fragment of QS-21
- PMID: 41064237
- PMCID: PMC12502733
- DOI: 10.3389/fchem.2025.1650302
A streamlined synthetic approach to the truncated linear trisaccharide fragment of QS-21
Abstract
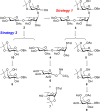
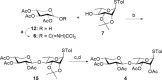
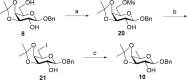
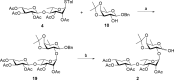
QS-21, a potent immunostimulatory saponin obtained from Quillaja saponaria Molina, a soapbark tree native to Chile, has undergone extensive study for its broad application as a vaccine adjuvant against various infectious diseases and cancers. The structure of QS-21, which features a linear oligosaccharide moiety, provides a critical attachment site for both the labile acyl side chain and the distinctive sugar unit that defines each major saponin variant. In this study, we present an efficient synthetic approach to the truncated linear trisaccharide fragment of QS-21, circumventing the challenges associated with the synthesis of the rare sugar D-fucose. The synthesis of this linear trisaccharide enables streamlined access to a homogeneous QS-21.
Keywords: QS-21; carbohydrate chemistry; glycosylation; linear trisaccharide; vaccine adjuvant.
Copyright © 2025 Lin, Tzeng, Dumalaog and Hung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures