A phase 2b basket trial approach to treat multiple rare and fibrotic skin diseases
- PMID: 41064516
- PMCID: PMC12500455
- DOI: 10.3389/fmed.2025.1637040
A phase 2b basket trial approach to treat multiple rare and fibrotic skin diseases
Abstract
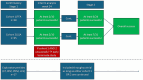
Fibrotic skin diseases are rare, chronic, and often debilitating conditions characterized by excessive extracellular matrix deposition, leading to tissue scarring and functional impairment. Despite their severity, diseases-such as lichen sclerosus et atrophicus (LSA), frontal fibrosing alopecia (FFA), radiation-induced skin fibrosis (RISF), eosinophilic fasciitis (EF), pansclerotic disabling morphea (PDM), and linear circumscript sclerodermia (LCS)-lack approved therapies and are underrepresented in clinical research. This phase 2b multicenter basket trial proposes a novel approach to evaluate a common antifibrotic therapy across these diverse but pathophysiologically related conditions. The trial employs a two-stage Simon design to address the statistical challenges posed by small patient populations, allowing the inclusion of ultra-rare diseases while maintaining analytical rigor. LSA and FFA serve as primary study groups due to higher prevalence, while EF, RISF, PDM, and LCS are included as exploratory arms. The study aims to assess the efficacy, safety, and tolerability of the selected therapy, while also providing mechanistic insights into fibrosis through molecular analyses. The primary endpoint is a ≥ 1-point improvement in the Investigator Global Assessment (IGA) at 24 weeks. Secondary endpoints at 52 weeks encompass quality of life (Dermatological Life Quality Index (DLQI), EuroQol Group Quality of Life Questionnaire (EuroQol five dimensions (EQ-5D))), symptom relief (itch and pain Numeric Rating Scale (NRS)), and disease-specific clinical scores. The trial excludes a placebo arm due to ethical considerations in progressive, untreated diseases but allows rescue therapies for disease progression. This design not only facilitates access to treatment for underserved populations but also leverages shared clinical and molecular features to enhance statistical power. By integrating disease-specific and global outcome measures, the study aims to generate robust evidence for repurposing existing therapies. If successful, this trial could serve as a model for future research in rare fibrotic diseases, accelerating drug development and improving patient outcomes.
Keywords: Simon design; basket trial; clinical score; clinical trial design; fibrotic skin diseases; rare diseases.
Copyright © 2025 Volc, Martus, Schefzyk, Günther, Moinzadeh, Susok, Ferrer, Zago and Pfeiffer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Handling editor DN declared a past authorship with the author LS.
Figures
References
-
- Meyer-Wilmes P, Wittenborn J, Kupec T, Caspers R, Stickeler E, Iborra S. Patient satisfaction and sexual issues in vulvar lichen sclerosus treatment: a monocentric certified dysplasia unit survey analysis. Arch Gynecol Obstet. (2024) 310:507–13. doi: 10.1007/s00404-024-07519-w, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

