Breathe easy, baby, breathe. Lung ultrasound in neonatal critical care
- PMID: 41064630
- PMCID: PMC12500432
- DOI: 10.3389/fped.2025.1631563
Breathe easy, baby, breathe. Lung ultrasound in neonatal critical care
Abstract
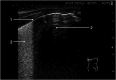
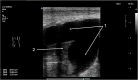
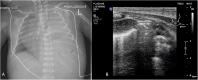
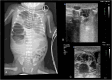
Lung ultrasound (LUS) has emerged as an essential tool in neonatology over the past two decades, offering unique advantages for this patient population. The small size, high water content, and delayed rib calcification of neonates make them particularly suited for ultrasonographic imaging. By replacing traditional chest radiographs, it significantly reduces exposure to ionizing radiation. Furthermore, it is widely accessible, easy to use, and provides repeatable, real-time imaging without requiring patient transport. These features make it invaluable in managing acute respiratory conditions, where timely intervention is critical. This review emphasizes the role of LUS in neonates with acute respiratory distress as a fundamental component of the point-of-care ultrasound (PoCUS) protocol. The technique is crucial for conditions such as respiratory distress syndrome (RDS), supporting decisions on surfactant therapy. It also aids in diagnosing and managing air-leak syndromes like pneumothorax (PTX) and detecting congenital respiratory malformations. Additionally, LUS ensures safer transport of critically ill neonates and optimizes mechanical ventilation. By delivering accurate, real-time imaging, LUS has become an essential diagnostic tool in infant care. Its integration into clinical practice enhances the management of life-threatening conditions, making it an essential skill for clinicians in neonatal intensive care units (NICU) and during neonatal transport.
Keywords: acute respiratory failure; lung ultrasound; neonatal transport; neonate; point-of care ultrasound.
© 2025 Jagła, Grudzień, Tomasik, Wroński and Kwinta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

