Co-expression of CD30 and SLFN11 serves as a dual biomarker for the treatment of cutaneous T-cell lymphoma
- PMID: 41064808
- PMCID: PMC12501776
- DOI: 10.1093/narcan/zcaf037
Co-expression of CD30 and SLFN11 serves as a dual biomarker for the treatment of cutaneous T-cell lymphoma
Abstract
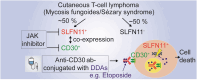
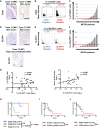
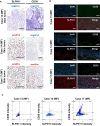
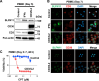
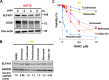
Advanced-stage cutaneous T-cell lymphoma (CTCL) is treated with diverse modalities, including DNA-damaging agents, anti-CD30 antibody-drug conjugates, and histone deacetylase (HDAC) inhibitors. Schlafen 11 (SLFN11) has emerged as a key determinant of sensitivity to DNA-damaging agents, yet its role in CTCL remains unclear. Here, we examined SLFN11 expression in two major CTCL subtypes-mycosis fungoides (MF) and Sézary syndrome (SS). Immunohistochemistry revealed SLFN11 positivity in 52% of MF (13/25) and 80% of SS (4/5) cases, with multivariate analysis showing a significant correlation between SLFN11 and CD30 expression. In normal human peripheral blood mononuclear cells, CD3/CD28/IL-2 stimulation induced co-expression of SLFN11 and CD30 in T cells, which was accompanied by heightened sensitivity to DNA-damaging agents. The JAK inhibitor cerdulatinib suppressed both markers. Among five CTCL cell lines, HUT78-expressing the highest SLFN11 levels-was the most sensitive to DNA-damaging agents, whereas SLFN11 knockout conferred resistance. Attempts to restore SLFN11 expression in SLFN11-low CTCL cells using six (pre)clinical HDAC inhibitors produced inconsistent results across cell lines and drugs. Together, these findings identify SLFN11 and CD30 as co-expressed therapeutic targets in CTCL and support the rationale for CD30-directed antibody-DNA-damaging agent conjugates as a precision treatment strategy.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures

References
-
- Board TWCoTE WHO Classification of Tumours. 2024; 5th ednLyon: IARC Press.
-
- Agar NS, Wedgeworth E, Crichton S et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010; 28:4730–9. 10.1200/JCO.2009.27.7665. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

