Adaptive Biomarker-Based Design for Early Phase Clinical Trials
- PMID: 41066076
- PMCID: PMC12510287
- DOI: 10.1002/sim.70275
Adaptive Biomarker-Based Design for Early Phase Clinical Trials
Abstract
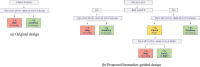
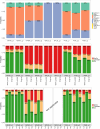
Identifying and quantifying predictive biomarkers is a critical issue of Precision Medicine approaches and patient-centric clinical development strategies. Early phase adaptive designs can improve trial efficiency by allowing for adaptations during the course of the trial. In this work, we are interested in adaptations based on interim analysis permitting a refinement of the existing study population according to their predictive biomarkers. At an early stage, the goal is not to precisely define the target population, but to not miss an efficacy signal that might be limited to a biomarker subgroup. In this work, we propose a one-arm two-stage early phase biomarker-guided design in the setting of an oncology trial where at the time of the interim analysis, several decisions can be made regarding stopping the entire trial early or continuing to recruit patients from the full or a selected patient population. Via simulations, we show that, although the sample size is limited, the proposed design leads to better decision-making compared to a classical design that does not consider an enrichment expansion.
Keywords: continuous biomarker; early phase; personalized medicine; predictive.
© 2025 The Author(s). Statistics in Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
J.G. and S.G. are the employees of Institut de Recherches Internationales Servier. G.S.‐H. is President of Saryga SAS. P.M. and A.S. served as statistical consultants for Institut de Recherches Internationales Servier and Saryga SAS.
Figures
References
-
- US Food and Drug Administration , “Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products,” 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
