Kidney-specific claudin-2 deficiency leads to medullary nephrocalcinosis in mice
- PMID: 41066193
- PMCID: PMC12646660
- DOI: 10.1172/JCI197807
Kidney-specific claudin-2 deficiency leads to medullary nephrocalcinosis in mice
Abstract
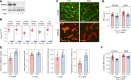
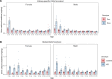
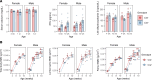
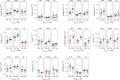
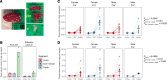
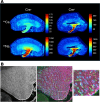
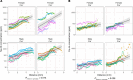
Deposits of hydroxyapatite called Randall's plaques are found in the renal papilla of calcium oxalate kidney stone formers and likely serve as the nidus for stone formation, but their pathogenesis is unknown. Claudin-2 is a paracellular ion channel that mediates calcium reabsorption in the renal proximal tubule. To investigate the role of renal claudin-2, we generated kidney tubule-specific claudin-2 conditional knockout mice (KS-Cldn2 KO). KS-Cldn2 KO mice exhibited transient hypercalciuria in early life. Normalization of urine calcium was accompanied by a compensatory increase in expression and function of renal tubule calcium transporters, including in the thick ascending limb. Despite normocalciuria, KS-Cldn2 KO mice developed papillary hydroxyapatite deposits, beginning at 6 months of age, that resembled Randall's plaques and tubule plugs. Bulk chemical tissue analysis and laser ablation-inductively coupled plasma mass spectrometry revealed a gradient of intrarenal calcium concentration along the corticomedullary axis in normal mice, that was accentuated in KS-Cldn2 KO mice. Our findings provide evidence for the "vas washdown" hypothesis for Randall's plaque formation, and identify the corticomedullary calcium gradient as a target for therapies to prevent kidney stone disease.
Keywords: Calcium; Epithelial transport of ions and water; Metabolism; Nephrology; Tight junctions.
Conflict of interest statement
Figures



References
-
- Bushinsky DA, et al. Nephrolithiasis. In: Taal MW, et al, eds. Brenner and Rector’s The Kidney. Elsevier; 2012:1455–1507.
LinkOut - more resources
Full Text Sources
Research Materials

