Correction: IRF6 controls Epstein-Barr virus (EBV) lytic reactivation and differentiation in EBV-infected epithelial cells
- PMID: 41066366
- PMCID: PMC12510547
- DOI: 10.1371/journal.ppat.1013564
Correction: IRF6 controls Epstein-Barr virus (EBV) lytic reactivation and differentiation in EBV-infected epithelial cells
Abstract
[This corrects the article DOI: 10.1371/journal.ppat.1013236.].
Copyright: © 2025 The PLOS Pathogens Staff. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Figures


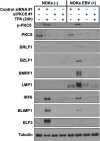



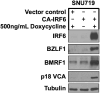

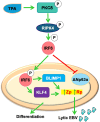
Erratum for
-
IRF6 controls Epstein-Barr virus (EBV) lytic reactivation and differentiation in EBV-infected epithelial cells.PLoS Pathog. 2025 Jun 26;21(6):e1013236. doi: 10.1371/journal.ppat.1013236. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40569988 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

