A Lyt at the end of the tunnel? Unraveling the complex interactions of the N-acetylglucosaminidase LytG in cell wall metabolism
- PMID: 41070187
- PMCID: PMC12505155
- DOI: 10.1039/d5cb00151j
A Lyt at the end of the tunnel? Unraveling the complex interactions of the N-acetylglucosaminidase LytG in cell wall metabolism
Abstract
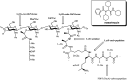
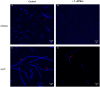
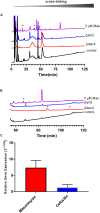
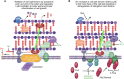
The growth and division of the Gram-positive cell requires the coordinated action of enzymes involved in the synthesis and degradation of the heteropolymer peptidoglycan. Herein, we present the use of the diamide masarimycin, an inhibitor of the exo-N-acetylglucosaminidase (GlcNAcase) LytG from Bacillus subtilis, as a chemical biology probe to elucidate the biological role of this cell wall degrading enzyme. Using a combination of chemical biology and genetic approaches we provide the first evidence that LytG activity influences the elongation and division complexes in B. subtilis. Chemical inhibition of LytG resulted in dysregulated cell elongation and localization of the division plane and the induction of the cell wall stress response. In the presence of masarimycin, cells show asymmetrical thickening of the cell wall and dysregulation of division plane localization. The use of genetic and synergy/antagonism screens established connections to late-stage peptidoglycan synthesis, particularly related to cross-linking function. These results stand in stark contrast to those observed for the ΔlytG knockout, which does not exhibit these phenotypes. Cell-wall labelling with a fluorescent d-amino acid and muropeptide analysis has highlighted a functional connection between LytG, the carboxypeptidase DacA, and d,d-endopeptidases. These results highlight the use of chemical probes such as masarimycin to inform on the biological function of autolysins by providing insight into the role LytG plays in cell growth and division.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures