Task-specific cortical mechanisms of taVNS-paired task-oriented training for post-stroke upper extremity dysfunction under cognitive load: an fNIRS study
- PMID: 41070188
- PMCID: PMC12504255
- DOI: 10.3389/fnhum.2025.1652612
Task-specific cortical mechanisms of taVNS-paired task-oriented training for post-stroke upper extremity dysfunction under cognitive load: an fNIRS study
Abstract
Objective: This study aimed to investigate the cortical task-specific response patterns underlying the improvement of upper limb dysfunction in stroke patients using transcutaneous auricular vagus nerve stimulation (taVNS) paired with task-oriented training (TOT) under varying cognitive loads.
Methods: In this randomized, double-blinded, sham-controlled trial, 30 patients with subacute stroke were enrolled and randomly assigned to either the taVNS group or the Sham group. Both groups received 3 weeks of TOT. The taVNS group received concurrent active taVNS, while the Sham group received concurrent sham stimulation. Assessments were performed pre- and post-intervention. Clinical function was evaluated using the Fugl-Meyer Assessment-Upper Extremity (FMA-UE), Montreal Cognitive Assessment (MoCA), Fatigue Severity Scale (FSS), and Modified Barthel Index (MBI). Neurophysiological measures included heart rate variability (HRV) to assess taVNS efficacy and motor-evoked potentials (MEPs) to assess cortical excitability changes. Brain functional imaging was conducted using functional near-infrared spectroscopy (fNIRS) during motor tasks with different cognitive loads (low-load: continuous horizontal movement; high-load: goal-directed movement) to analyze changes in spontaneous neural activity, task-related regional brain activation characteristics, and brain functional network alterations.
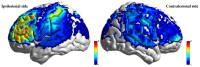
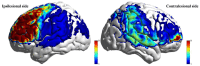
Results: (1) Post-intervention, the taVNS group showed significantly greater improvements in all HRV indices compared to the Sham group (P < 0.05). (2) Both groups exhibited significant improvements from baseline in FMA-UE, MoCA, MBI, and FSS scores (P < 0.05), with the taVNS group demonstrating significantly greater improvement than the Sham group (P < 0.05). (3) MEP results indicated significant improvements in the elicitation rate of ipsilesional MEPs within the taVNS group post-intervention (P < 0.05). Furthermore, compared to the Sham group, the taVNS group showed significantly greater improvements in the ipsilesional MEP elicitation rate and a significant reduction in contralesional MEP latency (P < 0.05). (4) Regarding resting-state fNIRS, the taVNS group exhibited higher Amplitude of Low-Frequency Fluctuation (ALFF) values post-intervention in the ipsilesional prefrontal cortex (PFC), dorsolateral prefrontal cortex (DLPFC), and sensorimotor cortex (SMC) compared to the Sham group (P < 0.05), but these differences were not significant after correction. In task-state fNIR under the low-cognitive-load condition, activation levels in the ipsilesionalS primary motor cortex (M1) and premotor and supplementary motor areas (pSMA) were significantly higher in the taVNS group compared to the Sham group post-intervention (P FDR < 0.05). During the high-cognitive-load task, activation levels in the ipsilesional PFC and DLPFC were significantly higher in the taVNS group compared to the Sham group post-intervention (P FDR < 0.05). (5) Functional network analysis using complex network metrics revealed that the taVNS group exhibited significantly increased nodal clustering coefficient and nodal local efficiency in the ipsilesional DLPFC during the high-cognitive-load task post-intervention compared to the Sham group (P FDR < 0.05).
Conclusion: taVNS paired with TOT enhances autonomic homeostasis, increases corticospinal pathway excitability, activates cognition-motor related brain regions, and modulates functional connectivity networks through multi-pathway neuroregulatory mechanisms. This promotes the formation of task-specific cortical activation and network connectivity during motor tasks under varying cognitive demands in stroke patients. These changes contribute to improved executive control performance in complex tasks, thereby enhancing cognitive-motor integration capabilities and facilitating upper limb functional recovery.
Clinical trial registration: https://www.chictr.org.cn/index.html, Unique Identifier/Registration Number: ChiCTR2400085163.
Keywords: functional near-infrared spectroscopy; motor-evoked potentials; neuroplasticity; task-oriented training; transcutaneous auricular vagus nerve stimulation; upper extremity rehabilitation.
Copyright © 2025 Li, Xu, Wang, Wang, Li, Lin and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Almhdawi K. A., Jaber H. B., Khalil H. W., Kanaan S. F., Shyyab A. A., Mansour Z. M., et al. (2021). Post-stroke fatigue level is significantly associated with mental health component of health-related quality of life: a cross-sectional study. Qual. Life Res. 30, 1165–1172. 10.1007/s11136-020-02714-z - DOI - PubMed
-
- An S., Oh S. J., Noh S., Jun S. B., Sung J. E. (2025). Enhancing cognitive abilities through transcutaneous auricular vagus nerve stimulation: findings from prefrontal functional connectivity analysis and virtual brain simulation. NeuroImage 311:121179. 10.1016/j.neuroimage.2025.121179 - DOI - PubMed
-
- Badran B. W., Peng X., Baker-Vogel B., Hutchison S., Finetto P., Rishe K., et al. (2023). Motor activated auricular vagus nerve stimulation as a potential neuromodulation approach for post-stroke motor rehabilitation: a pilot study. Neurorehab. Neural Repair 37, 374–383. 10.1177/15459683231173357 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

