Development of Biosimilar Aflibercept SDZ-AFL
- PMID: 41070355
- PMCID: PMC12504959
- DOI: 10.1016/j.curtheres.2025.100812
Development of Biosimilar Aflibercept SDZ-AFL
Abstract
Purpose: Aflibercept is a recombinant fusion protein that binds with high affinity to vascular endothelial growth factor A (VEGF-A), and other growth factors, reducing pathological neovascularization and abnormal vascular permeability. Aflibercept is approved as a first-line treatment for several retinal diseases, including neovascular age-related macular degeneration (nAMD); however, the high costs of these biologic agents can impede patient access. In 2024, Sandoz biosimilar aflibercept (SDZ-AFL; SOK583A1, Enzeevu/Afqlir) was approved by the US Food and Drug Administration and the European Medicines Agency as a biosimilar of reference aflibercept (Ref-AFL; Eylea, a trademark of Bayer AG and in the US of Regeneron Pharmaceuticals, Inc) based on a comprehensive package of data.
Methods: This narrative review summarizes the totality of evidence demonstrating biosimilarity between SDZ-AFL and Ref-AFL, including physicochemical and biological characterization data from analytical in vitro studies, results of an ocular pharmacokinetic (PK) study in rabbits, and clinical efficacy, safety, immunogenicity, and systemic PK data from Phase III clinical studies.
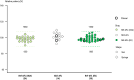
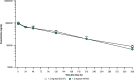
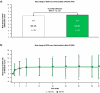
Results: Analytical evaluation demonstrated that SDZ-AFL has structural homology with Ref-AFL, including identical amino acid sequences, indistinguishable higher-order structures, and highly similar levels of structural variants. SDZ-AFL and Ref-AFL also demonstrated highly similar in vitro biologic activities, including target binding affinity, and potency in terms of neutralization of VEGF-A. In a single-dose ocular PK study in rabbits, vitreal exposure was comparable between SDZ-AFL and Ref-AFL after intravitreal administration. A 52-week Phase III clinical study (Mylight) evaluated the efficacy, safety, immunogenicity, and systemic PK of SDZ-AFL and Ref-AFL in 484 participants with nAMD. The primary endpoint was mean change from baseline to week 8 in best corrected visual acuity (BCVA): the difference between the SDZ-AFL and Ref-AFL groups in this endpoint was -0.3 letters (90% CI -1.5 to 1.0), which met predefined bioequivalence margins. Secondary efficacy endpoints, including changes from baseline in BCVA and central subfield foveal thickness over the whole 52-week study, were also similar for SDZ-AFL and Ref-AFL. Two subsequent "in-use" studies confirmed the safe use of SDZ-AFL provided in either a vial kit or a prefilled syringe.
Conclusion: This comprehensive totality of evidence has established biosimilarity between SDZ-AFL and Ref-AFL based on comparable physicochemical and biological characteristics, as well as similarity in clinical efficacy, safety, and immunogenicity. The introduction of aflibercept biosimilars to the market is anticipated to reduce barriers to access, potentially increasing the number of appropriate patients with retinal diseases benefiting from this biologic therapy.
Keywords: Aflibercept; Biosimilar; Neovascular age-related macular degeneration; Retinal disease; SOK583A1.
© 2025 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The presented research was sponsored by Sandoz. CW, DU, FD, JH, LA, and PA are employees of Sandoz AG or affiliates. RS has received consultancy funding from AbbVie, Bayer, Roche, and Théa Laboratories.
Figures
References
-
- Adams B.S., Sorhaitz W., Stringham J. StatPearls. StatPearls Publishing; Treasure Island (FL): 2025. Aflibercept.https://www.ncbi.nlm.nih.gov/pubmed/35881741 Accessed August 5, 2025.
-
- European Medicines Agency. Eylea Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/eylea-epar-pr.... Accessed August 5, 2025.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

