One-Year Outcomes After Traumatic Brain Injury and Early Extracranial Surgery in the TRACK-TBI Study
- PMID: 41071547
- PMCID: PMC12514633
- DOI: 10.1001/jamanetworkopen.2025.37271
One-Year Outcomes After Traumatic Brain Injury and Early Extracranial Surgery in the TRACK-TBI Study
Abstract
Importance: Exposure to extracranial (EC) surgery early after traumatic brain injury (TBI) is associated with cognitive risks.
Objective: To examine whether exposure to EC surgery during a TBI index admission is associated with worse outcomes at 1 year compared with no EC surgery.
Design, setting, and participants: This was a retrospective secondary nested cohort study of the prospective, observational Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) cohort study that enrolled participants from February 1, 2014, through August 31, 2018, at 18 US level I trauma centers. Participants aged 17 years or older who were admitted to an inpatient unit from the emergency department (ED) within 24 hours of trauma, had a known Glasgow Coma Scale (GCS) score and head computed tomography (CT) imaging, and did not undergo intracranial surgery were followed for up to 1 year after TBI and were analyzed for this study from July 25, 2023, to July 2, 2025.
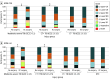
Exposure: Participants that underwent EC surgery during the index admission were compared with nonsurgical participants within the following injury subgroups: orthopedic trauma controls (OTCs), moderate-severe TBI (GCS 3-12), and computed tomography (CT) scan results that were positive (CT+) or negative (CT-) for acute intracranial findings along with a GCS score of 13 to 15.
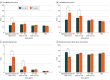
Main outcomes and measures: Brain injury-specific functional outcomes (Glasgow Outcome Scale-Extended [GOSE-TBI]), cognition (Trail Making Test [Trails] parts A and B), Disability Rating Scale (DRS), and Quality of Life After Brain Injury-Overall Scale (QOLIBRI-OS). A fixed-effects linear regression model with propensity weighting for missing outcome and group imbalance in baseline characteristics was used.
Results: Of the 1835 participants, 1279 (70%) were male, with mean (SD) age of 42.2 (17.8) years; 1349 participants (74%) were nonsurgical and 486 (26%) underwent EC surgery. In the 1150 participants (63%) followed up at 1 year, after propensity weighting, patients undergoing EC surgery in both the CT+ TBI and moderate-severe TBI subgroups had significantly worse GOSE-TBI (B, -0.57 [95% CI, -0.92 to -0.22] and -1.25 [95% CI, -1.65 to -0.85], respectively), Trails part B (B, 22.7 [95% CI, 7.4-38.1] and 47.9 [95% CI, 27.0-68.8]), and DRS (B, 2.47 [95% CI, 1.30-3.64] and 3.53 [95% CI, 2.19-4.87]) scores compared with nonsurgical participants. QOLIBRI-OS was worse after EC surgery vs no EC surgery in the subgroup with moderate-severe TBI (B, -15.1 [95% CI, -24.3 to -5.9]). There was no association of EC surgery with outcomes in the OTC or CT- TBI subgroups. For example, GOSE-TBI was not associated with EC surgery in the CT- TBI subgroup (B, 0.02 [95% CI, -0.24 to 0.27]).
Conclusions and relevance: In this cohort study, early EC surgery was associated with adverse function, cognition, and disability after TBI rated as moderate-severe or with radiographic abnormalities on CT scan regardless of GCS at index admission but not after orthopedic trauma or CT- TBI. Further studies may help determine whether surgical timing or other interventions can improve the observed long-term deficits.
Conflict of interest statement
Figures
References
-
- Reese M, Wong MK, Cheong V, et al. ; Markers of Alzheimer’s Disease and neuroCognitive Outcomes after Perioperative Care (MADCO-PC) Investigators . Cognitive and cerebrospinal fluid Alzheimer’s disease-related biomarker trajectories in older surgical patients and matched nonsurgical controls. Anesthesiology. 2024;140(5):963-978. doi: 10.1097/ALN.0000000000004924 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

