Activated CaMKIIδ translocates to the RyR nanodomain in cardiomyocytes
- PMID: 41071938
- PMCID: PMC12638726
- DOI: 10.1093/cvr/cvaf187
Activated CaMKIIδ translocates to the RyR nanodomain in cardiomyocytes
Abstract
Aims: The heartbeat is triggered by the coordinated release of Ca2+ from the ryanodine receptor type-2 (RyR) in cardiomyocytes. Phosphorylation of RyR by Ca2+/calmodulin-dependent kinase IIδ (CaMKIIδ) fine-tunes this process in health, while hyperphosphorylation causes excessive, pathological Ca2+ release. We investigated how CaMKIIδ is spatially recruited and anchored to RyRs to achieve this functional regulation.
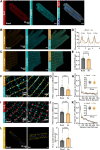
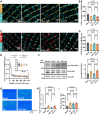
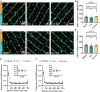
Methods and results: We employed confocal and dSTORM microscopy to investigate the macro- and nanoscale distribution of CaMKIIδ across cardiomyocytes, respectively. We linked positional rearrangement of the kinase during β-adrenergic stimulation (isoproterenol, Iso) to alterations in RyR phosphorylation and function (Ca2+ sparks), and the requirement of the CaMKIIδ anchoring protein AKAP18δ by knockdown/knockout. Confocal microscopy revealed that macroscale CaMKIIδ localization was not markedly altered during Iso-treatment, although a narrowing of its distribution around the Z-lines occurred, where the RyR reside. Higher resolution dSTORM imaging confirmed that local mobilization of CaMKIIδ by Iso decreased the distance from Z-lines and RyRs to the nearest CaMKIIδ by 28 and 12%, respectively. Functionally, kinase translocation into the RyR nanodomain was accompanied by increased channel phosphorylation and Ca2+ spark frequency. These actions were dependent on CaMKIIδ activity, since kinase translocation, RyR phosphorylation, and activation were all mimicked by the upstream activator of CaMKIIδ (8-CPT) and prevented by direct CaMKIIδ inhibitors (AIP, N1 peptide). A critical role of AKAP18δ in this mechanism was supported by immunoprecipitation experiments, which showed greater kinase binding to AKAP18δ during Iso-stimulation. Furthermore, loss of AKAP18δ by viral-mediated AKAP18δ knockdown or knockout prevented CaMKIIδ translocation to Z-lines. Microtubular disruption also blocked CaMKIIδ translocation.
Conclusion: Collectively, our results indicate that nanoscale movement of CaMKIIδ is closely associated with RyR activation following β-adrenergic stimulation. This translocation depends on an intact microtubular network and kinase binding to AKAP18δ.
Keywords: AKAP18δ; Ca(2+) homeostasis; CaMKIIδ; Cardiomyocyte; RyR.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures



Comment in
-
Kinases go where the function calls.Cardiovasc Res. 2025 Nov 22;121(14):2116-2117. doi: 10.1093/cvr/cvaf197. Cardiovasc Res. 2025. PMID: 41118147 No abstract available.
References
-
- Bers DM. Cardiac excitation–contraction coupling. Nature 2002;415:198–205. - PubMed
-
- Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation–contraction coupling in the heart. Cardiovasc Res 2007;73:631–640. - PubMed
-
- Rosenberg OS, Deindl S, Sung R-J, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell 2005;123:849–860. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

