Rituximab-induced long-term remission in childhood-onset, uncomplicated, frequently relapsing or steroid-dependent nephrotic syndrome: a randomized, placebo-controlled trial and a follow-up study
- PMID: 41073503
- PMCID: PMC12514064
- DOI: 10.1038/s41598-025-19214-0
Rituximab-induced long-term remission in childhood-onset, uncomplicated, frequently relapsing or steroid-dependent nephrotic syndrome: a randomized, placebo-controlled trial and a follow-up study
Abstract
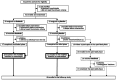
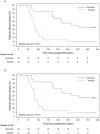
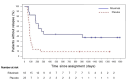
Rituximab maintains remission of complicated frequently relapsing or steroid-dependent nephrotic syndrome (FRNS/SDNS) by depleting peripheral B cells, but most patients eventually experience relapses after B cell recovery. We performed a multicenter, double-blind, randomized, placebo-controlled trial to assess rituximab's efficacy and safety for childhood-onset uncomplicated FRNS/SDNS (without prior treatment with glucocorticoid-sparing immunosuppressive agents) with a follow-up study to assess rituximab's long-term effect after B cell recovery. Patients were randomly assigned to receive either rituximab (375 mg/m2, maximum 500 mg, once weekly for 2 weeks) or placebo. The primary endpoint was the relapse-free period. Of 43 randomized patients, 40 received the intervention (18 rituximab, 22 placebo). The relapse-free period during the 1-year trial was significantly longer in the rituximab vs. placebo groups (median: 285 vs. 81 days; p < 0.001). Infusion reactions were more frequent in the rituximab group (p < 0.001), with no difference in adverse events incidence between the groups. Interestingly, the follow-up study demonstrated markedly higher 3-year cumulative relapse-free survival probability without further treatments in the rituximab vs. placebo groups (38% vs. 9%). A mini-systematic review with meta-analyses supported the findings. Rituximab is effective and well-tolerated, potentially leading to long-term remission with substantially high rates after B cell recovery for childhood-onset uncomplicated FRNS/SDNS.Trial registration JSKDC10, Clinical Trials Registry ID: jRCT1091220380; JSKDC10 follow-up study, Clinical Trials Registry ID: jRCT1050230024.
Keywords: Children; Long-term remission; Rituximab; Uncomplicated frequently relapsing or steroid-dependent nephrotic syndrome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: KIi reports a research grant for clinical trials and clinical research from Zenyaku Kogyo Co. Ltd. to his institution; consulting fees from Kyowa Kirin Co. Ltd., and Chugai Pharmaceutical Co., Ltd.; honoraria for manuscript writing from Zenyaku Kogyo Co. Ltd.; payment for expert testimony to my institution from Zenyaku Kogyo Co. Ltd.; and serves as a member of the advisory board of Kyowa Kirin Co. Ltd. and Chugai Pharmaceutical Co., Ltd.. MS reports grants for clinical trials and development promotion network from Japan Agency for Medical Research and Development; honoraria for lectures from Chugai Pharmaceutical Co., Ltd.; payment for expert testimony to her institution from Zenyaku Kogyo Co. Ltd.; and patent application of agent for treatment of pediatric hypozincemia. TH reports grants for clinical research from Sysmex Corporation, Otsuka Pharmaceutical Co. Ltd., and Zenyaku Kogyo Co. Ltd.. TS has nothing to disclose. TY has nothing to disclose. RH has nothing to disclose. YO reports honoraria for lectures from ARKRAY, Inc., Merck & Co., Inc., and SHIONOGI & CO., LTD.. ST has nothing to disclose. KK reports grants for clinical research from Public Foundation of Vaccination Research Center, Taiju Life Social Welfare Foundation, Chugai Pharmaceutical Co. Ltd., Teijin Pharma Ltd., Kyowa Kirin Co. Ltd., Taiho Pharmaceutical Co. Ltd., Shionogi & Co. Ltd., Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Co. Ltd., and Otsuka Pharmaceutical Co. Ltd.; honoraria for lectures from Terumo Co. Ltd., Baxter Ltd., Zenyaku Kogyo Co. Ltd.. RT has nothing to disclose. YK has nothing to disclose. TK has nothing to disclose. YS has nothing to disclose. TM has nothing to disclose. AI has nothing to disclose. SF has nothing to disclose. MO has nothing to disclose. HK has nothing to disclose. AK has nothing to disclose. CN has nothing to disclose. KNa reports consulting fees from Kyowa Kirin Co. Ltd. and AstraZeneca; honoraria for lectures from Kyowa Kirin Co. Ltd. and Novartis; and serves as the president of the Japanese Society for Pediatric Nephrology. KIs reports grants for clinical research from Asahi Kasei Pharma Corporation, Chugai Pharmaceutical Co., Ltd., Novartis International AG, Japan Blood Products Organization, Teijin Pharma Limited, and Astellas Pharma Inc.; consulting fee from Chugai Pharmaceutical Co., Ltd.; honoraria for lectures from Zenyaku Kogyo Co. Ltd., Novartis International AG, and Teijin Pharma Limited. SI reports grants from Zenyaku Kogyo, Co., Ltd., Chuigai Pharmaceutical Co. Ltd., Astellas Pharma Inc., and Asahi Kasei Pharma Corporation; consulting fee from Zenyaku Kogyo Co. Ltd.; honoraria from Zenyaku Kogyo, Co., Ltd., Chuigai Pharmaceutical Co. Ltd., Novartis International AG, Asahi Kasei Pharma Corporation, and Astellas Pharma Inc.. HN reports grants for regulatory science on facilitating pediatric drug development from Japan Agency for Medical Research and Development; consulting fees from Tohoku University, Daiichi Sankyo Company, Limited, and Pfizer R&D Japan G.K.; Honoraria for lectures from Japan Pharmaceutical Manufacturers Association, Pfizer Global Supply Japan Inc., Bristol Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., and Northern Science Consulting Inc.; and serves as a member of advisory board of Taisho Pharmaceutical Holdings Co., Ltd. for drug development for diabetes, a member of advisory board of Sato Pharmaceutical Co., Ltd. for drug development for dermatology, Chairman of the Committee on Pharmaceutical Affairs of Japan Pediatric Society, and Chairman of the Steering Committee of Japan Society on Developmental Pharmacology and Therapeutics. GMG has nothing to disclose. RM has nothing to disclose. TO has nothing to disclose. KN reports a research grant for clinical trials and clinical research from Zenyaku Kogyo Co. Ltd. to his institution; consulting fees from Kyowa Kirin Co. Ltd., Toa Eiyo LTD., Zenyaku Kogyo Co. Ltd., and Taisho Pharmaceutical Co. Ltd.; honoraria for lectures from Ono Pharma, Astellas Pharma, Novo Nordisk Pharma, Alexion Pharma, Sumitomo Pharma, Sanofi, Otsuka Pharma, Daiichi Sankyo, and Miyarisan; and serves as a member of the advisory board of Kyowa Kirin Co. Ltd..
Figures


References
-
- Iijima, K., Swiatecka-Urban, A., Niaudet, P. & Bagga, A. Steroid-sensitive nephrotic syndrome. In Pediatric Nephrology (eds Emma, F. et al.) 351–386 (Springer, Berlin, 2022). 10.1007/978-3-030-52719-8_92.
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. Suppl.100, S1–S276 (2021). 10.1016/j.kint.2021.05.021. - PubMed
-
- Vivarelli, M., Gibson, K., Sinha, A. & Boyer, O. Childhood nephrotic syndrome. Lancet402, 809–824. 10.1016/S0140-6736(23)01051-6 (2023). - PubMed
-
- Yadav, M., Sinha, A., Khandelwal, P., Hari, P. & Bagga, A. Efficacy of low-dose daily versus alternate-day prednisolone in frequently relapsing nephrotic syndrome: An open-label randomized controlled trial. Pediatr. Nephrol.34, 829–835. 10.1007/s00467-018-4071-7 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

