Constructing biomimetic microenvironments for liver regeneration
- PMID: 41074125
- PMCID: PMC12514811
- DOI: 10.1186/s12951-025-03729-9
Constructing biomimetic microenvironments for liver regeneration
Abstract
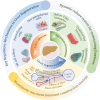
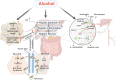
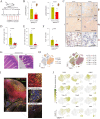
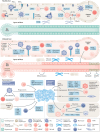
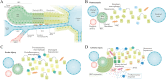
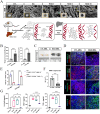
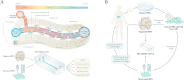
Liver regeneration is a sophisticated biological process influenced by a complex microenvironment that becomes profoundly altered in various pathological conditions. Current therapeutic approaches, including liver transplantation and pharmacological interventions, face significant limitations such as donor shortages, high costs, immune rejection, and insufficient functional recovery. Thus, alternative and innovative strategies are urgently needed. Biomimetic microenvironments constructed through tissue engineering have emerged as promising platforms, capable of recapitulating the liver's natural architecture and supporting hepatic cell functions. This review outlines key pathological features and the biological basis underlying liver regeneration, highlighting cellular plasticity, inflammation, extracellular matrix (ECM) remodeling, and immune interactions. It further discusses advanced biomimetic strategies, including 3D cell cultures, decellularized ECM hydrogels, bioprinting technologies, and dynamic culture systems like hollow fiber, fluidized-bed, and microcarrier bioreactors. These innovations facilitate accurate modeling of hepatic functions, maintain cellular differentiation, and enhance regeneration. Despite significant advancements, challenges remain in optimizing microenvironmental fidelity, ensuring clinical scalability, and translating laboratory breakthroughs into effective therapies.
Keywords: Biomaterials; Biomimetic microenvironment; Extracellular matrix; Liver diseases; Liver regeneration; Tissue engineering.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors gave their consent for publication. Competing interests: The authors declare no competing interests.
Figures





References
-
- Matchett KP, Wilson-Kanamori JR, Portman JR, Kapourani CA, Fercoq F, May S, Zajdel E, Beltran M, Sutherland EF, Mackey JBG, Brice M, Wilson GC, Wallace SJ, Kitto L, Younger NT, Dobie R, Mole DJ, Oniscu GC, Wigmore SJ, Ramachandran P, Vallejos CA, Carragher NO, Saeidinejad MM, Quaglia A, Jalan R, Simpson KJ, Kendall TJ, Rule JA, Lee WM, Hoare M, Weston CJ, Marioni JC, Teichmann SA, Bird TG, Carlin LM, Henderson NC. Multimodal decoding of human liver regeneration. Nature. 2024;630(8015):158–65. - PMC - PubMed
-
- Michalopoulos GK. Novel insights into liver homeostasis and regeneration. Nat Rev Gastroenterol Hepatol. 2021;18(6):369–70. - PubMed
-
- Scoditti E, Sabatini S, Carli F, Gastaldelli A. Hepatic glucose metabolism in the steatotic liver. Nat Rev Gastroenterol Hepatol. 2024;21(5):319–34. - PubMed
-
- Starling S. Obesity dysregulates a pituitary-liver axis through disruption of the unfolded protein response. Nat Rev Endocrinol. 2024;20(7):385. - PubMed
-
- Li Z, Peng W, Zhou J, Shui S, Liu Y, Li T, Zhan X, Chen Y, Lan F, Ying B, Wu Y. Multidimensional interactive cascading nanochips for detection of multiple liver diseases via precise metabolite profiling. Adv Mater. 2024;36(21):e2312799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

