Interferon-sensitized hematopoietic progenitors dynamically alter organismal immunity
- PMID: 41076641
- PMCID: PMC12614298
- DOI: 10.1093/jimmun/vkaf249
Interferon-sensitized hematopoietic progenitors dynamically alter organismal immunity
Abstract
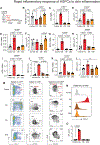
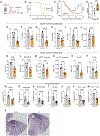
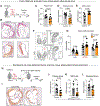
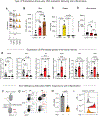
Inflammation has enduring impacts on organismal immunity. However, the precise mechanisms by which tissue-restricted inflammation conditions systemic responses are poorly understood. Here, we leveraged a highly compartmentalized model of skin inflammation and identified a surprising type I interferon (IFN)-mediated activation of hematopoietic stem/progenitor cells (HSPCs) that results in profound changes to systemic host responses. Post-inflamed mice were protected from atherosclerosis and had worse outcomes following influenza virus infection. This IFN-mediated HSPC modulation was dependent on IFNAR signaling and could be recapitulated with the administration of recombinant IFN-α. Importantly, the transfer of post-inflamed HSPCs was sufficient to transmit the immune suppression phenotype. IFN modulation of HSPCs was rooted both in long-term changes in chromatin accessibility and the emergence of an IFN-responsive functional state from multiple progenitor populations. Collectively, our data reveal the profound and enduring effect of transient inflammation and more specifically type I IFN signaling and set the stage for a more nuanced understanding of HSPC functional modulation by peripheral immune signals.
Keywords: hematopoietic stem and progenitor cells; innate immune memory; interferon α; skin inflammation.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
SN is on the SAB of Seed Inc. and receives funding from Takeda Pharmaceuticals. This activity is not relevant to the content of this manuscript. The remaining authors declare no competing interests.
Figures

Update of
-
Interferon-sensitized hematopoietic progenitors dynamically alter organismal immunity.bioRxiv [Preprint]. 2024 Apr 28:2024.04.24.590828. doi: 10.1101/2024.04.24.590828. bioRxiv. 2024. Update in: J Immunol. 2025 Oct 12:vkaf249. doi: 10.1093/jimmun/vkaf249. PMID: 38712060 Free PMC article. Updated. Preprint.
References
Grants and funding
- TL1 TR001447/TR/NCATS NIH HHS/United States
- K99 HL151963/HL/NHLBI NIH HHS/United States
- 20SFRN35210936/American Heart Association
- 1RO1CA283574/GF/NIH HHS/United States
- S10 OD021747/CD/ODCDC CDC HHS/United States
- P30CA016087/Cancer Center Support
- NYSCF Robertson Stem Cell Investigator
- S10 OD021747/OD/NIH HHS/United States
- R01-AI168462/NH/NIH HHS/United States
- Harold S. Geneen Charitable Trust
- HL131481/GF/NIH HHS/United States
- EvansMDS Foundation
- UWSC12146/National Heart, blood and Lung Institute
- P30 CA016087/CA/NCI NIH HHS/United States
- Packard Fellow
- Taub Foundation
- 1RO1HL159175/GF/NIH HHS/United States
- R00 HL151963/HL/NHLBI NIH HHS/United States
- P01 HL131481/HL/NHLBI NIH HHS/United States
- R01 AI168462/AI/NIAID NIH HHS/United States
- 00034119/Pew Stewert Scholar Award
- TL1 TR001447/NH/NIH HHS/United States
- R01 CA283574/CA/NCI NIH HHS/United States
- 1DP2AR079173-01/NH/NIH HHS/United States
- DP2 AR079173/AR/NIAMS NIH HHS/United States
- ALTF 742-2020/NH/NIH HHS/United States
- 18POST34080390/American Heart Association
- R01 HL159175/HL/NHLBI NIH HHS/United States
- NPF/National Psoriasis Foundation/United States
- HL151963/GF/NIH HHS/United States
- Laura and Isaac Perlmutter Cancer Center
- 1RO1CA271455/GF/NIH HHS/United States
- Vogelstein Family Foundation
- R01 CA271455/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources

