Respiratory syncytial virus (RSV) vaccine effectiveness and antibody correlates of protection among older adults in the Community Vaccine Effectiveness (CoVE) observational study
- PMID: 41076991
- PMCID: PMC12547470
- DOI: 10.1016/j.ebiom.2025.105961
Respiratory syncytial virus (RSV) vaccine effectiveness and antibody correlates of protection among older adults in the Community Vaccine Effectiveness (CoVE) observational study
Abstract
Background: The first RSV vaccines for adults 60 years and older were approved prior to the 2023-2024 respiratory virus season. This study aims to evaluate RSV vaccine effectiveness (VE) in preventing RSV infections among older adults, and to examine antibody correlates of protection.
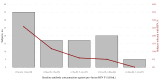
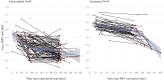
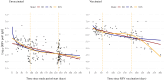
Methods: This study used data from adults 60 years and older, enrolled into the Community Vaccine Effectiveness (CoVE) prospective cohort study, in Michigan, U.S.A. A Cox regression model was used to compare incidence of symptomatic/all RSV infections in those vaccinated versus unvaccinated. RSV-specific (preF) binding antibodies were measured in serum specimens and assessed longitudinally. A correlates of protection analysis was conducted using logistic regression.
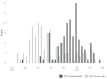
Findings: Of the 281 participants (n = 117 vaccinated) enrolled (August 1, 2023, to March 1, 2024), 14 tested positive for RSV. Adjusted RSV VE against any RSV infection was 50.8% (95% CI: -79.1% to 86.5%), and 59.8% (95% CI: -105.2% to 92.1%) against symptomatic RSV. There were 61.2 (95% CI: 16.9, 163.2) RSV infections per 1000 person-years among participants who were vaccinated compared to 165.8 infections (95% CI: 88.0, 287.0) per 1000 person-years among those unvaccinated. A 31% decrease in odds (OR: 0.69, 95% CI: 0.44-1.07) of RSV infection per 2-fold increase in antibody concentration was observed.
Interpretation: Our findings suggest that higher antibody levels may be associated with a reduced risk of RSV infection, but further research is needed to confirm this relationship. RSV incidence appeared to be lowest among adults who were vaccinated, though the difference was not statistically significant. Low number of RSV events and limited availability of serology data limit the precision of the estimates. Continued monitoring of reduction of RSV infection in years following vaccination is warranted.
Funding: National Center for Immunisation and Respiratory Diseases, U.S. Centers for Disease Control and Prevention (75D30122C13149) and National Institute of Allergy and Infectious Diseases (75N93021C00015). The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Keywords: Antibody waning; Cohort study; Correlates of protection; Older adults; Respiratory syncytial virus; Vaccine effectiveness.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests ASL received consulting fees from Roche related to the baloxavir clinical trial. All other authors declare no competing interests.
Figures
References
-
- Van Effelterre T., Hens N., White L.J., et al. Modeling respiratory syncytial virus adult vaccination in the United States with a dynamic transmission model. Clin Infect Dis. 2023;77(3):480–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

