Chitosan Derivatives Associated with Ceftazidime: Study of Controlled Release and Antibacterial Activity
- PMID: 41078762
- PMCID: PMC12509009
- DOI: 10.1021/acsomega.5c06901
Chitosan Derivatives Associated with Ceftazidime: Study of Controlled Release and Antibacterial Activity
Abstract
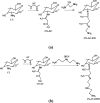
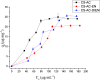
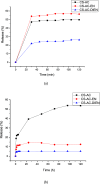
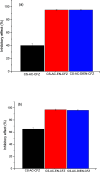
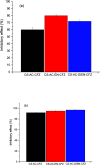
The search for new low-cost and nontoxic materials with the potential for controlled drug release and antibacterial activity has intensified recently. Due to their unique properties, chitosan derivatives have emerged as promising candidates for these applications. This study evaluated the ability of three chitosan derivatives to incorporate and control the release of the ceftazidime (CFZ) drug, as well as to investigate the antibacterial activity of these derivatives when combined with CFZ against Staphylococcus aureus and Escherichia coli. Initially, the incorporation of the CFZ drug into chitosan derivatives obtained via sequential modification with acetylacetone (CS-AC) and subsequently with ethylenediamine (CS-AC-EN) or diethylenetriamine (CS-AC-DIEN) was evaluated. The adsorption isotherms were best described by the Temkin model. The maximum adsorption capacities were 24.00 ± 0.20 μg mg- 1 (CS-AC), 20.10 ± 0.70 μg mg- 1 (CS-AC-EN), and 25.50 ± 0.50 μg mg- 1 (CS-AC-DIEN). In gastric medium, the chitosan derivatives exhibited rapid CFZ release within the first 30 min: 47.06 ± 0.80% (CS-AC), 53.84 ± 0.30% (CS-AC-EN), and 21.87 ± 0.60% (CS-AC-DIEN). By contrast, at intestinal pH, the release was slower and controlled, reaching 50.38 ± 0.10% for CS-AC at 72 h, 11.88 ± 0.50% for CS-AC-EN at 24 h, and 5.08 ± 0.30% for CS-AC-DIEN at 6 h. The release profiles were best fitted by the Korsmeyer-Peppas kinetic model. Antibacterial assays against S. aureus and E. coli showed that all three chitosan derivatives combined with CFZ outperformed CFZ alone and the mixture of unmodified chitosan with CFZ. The advantage was most pronounced after 72 h: against E. coli, all derivatives achieved >90% inhibition. These findings indicate that the chitosan derivatives are promising materials for controlling CFZ release in gastric and intestinal environments. Furthermore, when combined with CFZ, these derivatives demonstrate significant potential for antibacterial applications against Gram-positive and Gram-negative bacteria.
© 2025 The Authors. Published by American Chemical Society.
Figures
References
-
- Nezamoleslami S., Fattahi A., Nemati H., Bagrezaie F., Pourmanouchehri Z., Kiaie S. H.. Electrospun sandwich-structured of polycaprolactone/gelatin-based nanofibers with controlled release of Ceftazidime for wound dressing. Int. J. Biol. Macromol. 2023;236:123819. doi: 10.1016/j.ijbiomac.2023.123819. - DOI - PubMed
-
- Moreno A. H., Salgado H. R. N.. Development and validation of the quantitative analysis of Ceftazidime in powder for injection by infrared spectroscopy. Phys. Chem. 2012;2:6–11. doi: 10.5923/j.pc.20120201.02. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
