Quantitative videodensitometric assessment of aortic regurgitation in Myval, Sapien, and Evolut THV series: Results from the LANDMARK trial
- PMID: 41079007
- PMCID: PMC12512974
- DOI: 10.1016/j.ijcha.2025.101804
Quantitative videodensitometric assessment of aortic regurgitation in Myval, Sapien, and Evolut THV series: Results from the LANDMARK trial
Abstract
Background: The quantitative videodensitometric aortography (QVDA) has reliably quantified post-TAVI aortic regurgitation (AR). However, this method has not yet been evaluated in randomized trials comparing various transcatheter heart valve (THV) systems. Here, we investigated the QVDA of AR following TAVI for severe aortic stenosis among Myval, Sapien, and Evolut THV series as part of the LANDMARK trial.
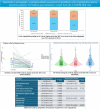
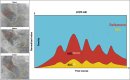
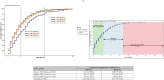
Methods: The final aortograms, either without or after balloon post-dilatation (BPD) were analyzed using the advanced CAAS-A-Valve 2.1.2 software. The regurgitant fraction (RF) was computed and categorized into none/trace AR (RF < 86 %), mild AR (6 % ≤ 8RF ≤ 817 %), and moderate/severe AR (RF > 17 %).
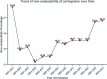
Results: Five hundred ninety-six final analyzable aortograms and 97 aortograms following BPD were included in the analysis. The BPD resulted in a significant reduction of RF in the Myval [12.0(6.0-18.5) vs 2.0(1.0, 5.5);p = 0.0002], Sapien[18.0(1.0-19.0) vs. 2.0(1.0-3.0); p = 0.04206] and Evolut [10.5 (6.0-15.0) vs 5.0 (1.0-8.0); p = 0.0009]. The rate of final RF > 17 % was lower in the Myval(2.0 %) compared to Evolut(8.00 %), but similar to the Sapien series (4.0 %)(PMyval-Sapien = 0.2333, PMyval-Evolut = 0.0057). In the as-treated population, the Myval series demonstrated a comparable RF to the Sapien series, but a significantly lower RF compared to the Evolut [Myval: 3.0 %(1.0-7.0), Sapien:3.0 %(1.0-7.0), Evolut:5.0 %(1.0-10.0)], PMyval-Sapien = 0.8997,PMyval-Evolut = 0.0010].
Conclusion: The QVDA highlights the superior performance of the Myval THV series compared to the Evolut THV series, with the lowest rate of moderate/severe RF among the three THV series, and could be used with echocardiography to help in detecting cases with none/trace AR.
Keywords: Aortic regurgitation; Balloon post-dilatation; Regurgitant fraction; Transcatheter aortic valve implantation; Videodensitometry.
© 2025 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Osama Soliman reports research grants from Biosensors, Boston Scientific, Cardiawave and Meril Life Sciences. P.W. Serruys reports consultancy fees from SMT, Novartis, Meril Life Sciences, and Philips. N. van Royen reports grant funding and personal fees from Abbott; grants from Philips, Biotronik, and Medtronic; and speaker fees from MicroPort, Bayer, and RainMed Medical outside the submitted work. I.J. Amat-Santos reports being a proctor for Medtronic, Boston Scientific, and Meril Life Sciences. A. Ijsselmuiden reports institutional fees from Medtronic and Abbott; consulting fees from Meril Life Sciences, Angiocare, Abbott, Philips, and Translumina. P. Laanmets received travel support from Meril Life Sciences to attend the conference. D. Unic reports payment/honoraria from Meril Life Sciences, Medtronic and abbott; and a member of the Medtronic EMEA surgical advisory board. B. Merkely reports institutional grants and speaker fees from Boehringer Ingelheim, DUKE Clinical Institute, and Novartis; institutional fees from Biotronik and Eli Lilly; direct personal payment from Daiichi Sankyo; national leader for Librexia program, New Amsterdam trial, DAPA ACT HF-TIMI 68 trial, FINEARTS-HF trial, REALIZE-K trial, SOS-AMI trial, DELIVER trial, GARDEN-TIMI 74 trial, ENDEAVOR trial, EMPACT-MI trial, CARDINAL-HF trial; rector of Semmelweis University, Director and chair of the Heart and Vascular Center of Semmelweis University. R.S. Hermanides reports speaker fees from Novartis, Edwards Life Sciences, Meril and Abbott vascular outside the submitted work. P. Martin reports proctorship grant from Meril Life Sciences; payment or honoraria for lectures, presentations from Meril Life Sciences, Boston Scientific Iberica, Abbott; Advisory board member for Medtronic Spain. M. De Sousa Almeida reports lecture fees from Medtronic and Novartis; travel support from Medtronic, Terumo and Boston Scientific. A. Linke received grants from Edward Lifesciences and Novartis; speaker honoraria from Edward Lifesciences, Boston Scientific, AbioMed, Pfizer, Astra Zeneca, Boehringer, Abbott, MSD, Corvia, Daiichi, and Meril; travel support from Meril, AbioMed and abbott; Stock option holder with Picardia, Transverse Medical and Filterlex. Alfonso Ielasi reports consulting fees, payment/honoraria for lectures, presentations from Meril Life Sciences, Sahajanand Medical Technologies and Cardionovum. K. Toutouzas reports proctorship with Abbott, Meril and Medtronic; consulting fee from Gore Medical; Board member Hellenic Society of Cardiology. F. Bedogni reports proctorship and consulting fees from Meril Life Sciences and Medtronic. Dolores Mesa Rubio reports minor lecture fees from Edwards and Abbott. O. Angerås reports proctorship and speaker fees from Meril Life and Abbott Medical; speaker fees from Medtronic; research grant from Abbott. W.-K. Kim reports honoraria or consultancy fees from Edwards Lifesciences, Boston Scientific, Meril Life Sciences, JenaValve, Abbott and P&F; advisory board member for P&F. J. Rothe reports personal fees for consulting/proctoring from Meril Life Sciences, Medtronic, Abbott and Qatna; and travel support for attending meetings from Meril Life Sciences, Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. A Thakkar, and U Chandra are full employees of Meril Life Sciences. P.W. Serruys reports consultancy fees from SMT, Novartis, Meril Life Sciences, and Philips. A. Baumbach reports consultation fees from Meril Life Sciences, Biotronik and Jenavalve; Lecture fees or honoraria from Biotronik; participation in DSMB for Pi Cardia and Faraday. The other authors have no conflicts of interest to declare.].
Figures





References
-
- Adams David H., Popma Jeffrey J., Reardon Michael J., Yakubov Steven J., Coselli Joseph S., Deeb G.M., et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. New Engl. J. Med. 2014;370(19):1790–1798. - PubMed
-
- Baumbach A., van Royen N., Amat-Santos I.J., Hudec M., Bunc M., Ijsselmuiden A., et al. LANDMARK comparison of early outcomes of newer-generation Myval transcatheter heart valve series with contemporary valves (Sapien and Evolut) in real-world individuals with severe symptomatic native aortic stenosis: a randomised non-inferiority trial. Lancet. 2024;403(10445):2695–2708. - PubMed
-
- Sondergaard L., Walton A.S., Worthley S.G., Smith D., Chehab B., Manoharan G., et al. Thirty-day and one-year outcomes of the Navitor transcatheter heart valve in patients with aortic stenosis: the prospective, multicentre, global PORTICO NG Study. EuroIntervention. 2023;19(3):248–255. - PubMed
-
- Tamburino C., Capodanno D., Ramondo A., Petronio A.S., Ettori F., Santoro G., et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123(3):299–308. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

