Metabolic dysregulation in the heart in obesity-associated HFpEF
- PMID: 41079580
- PMCID: PMC12511046
- DOI: 10.3389/fcvm.2025.1678992
Metabolic dysregulation in the heart in obesity-associated HFpEF
Abstract
Background: Obesity and hypertension are among the most prevalent comorbidities in heart failure with preserved ejection fraction (HFpEF). In addition to its relationship with hypertension in HFpEF, obesity is also strongly associated with insulin resistance (IR) and type 2 diabetes (T2D). However, the exact cardiac effects underlying this relationship are unknown. We sought to differentiate the cardiac phenotype associated with increased adiposity in the presence or absence of IR in obese HFpEF. We utilized adipose tissue-specific MitoNEET transgenic mice, which develop chronic, metabolically healthy adipose tissue expansion (obese non-insulin resistant, OB-NIR), and compared them with their wild-type, insulin-resistant littermates (OB-IR).
Methods: OB-NIR MitoNEET and OB-IR wildtype mice were fed a high-fat diet for 16 weeks, at which time HFpEF was induced via uninephrectomy, d-aldosterone infusion, and 1.0% sodium chloride drinking water for 4 additional weeks while maintained on the same diet.
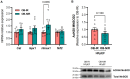
Results: OB-NIR HFpEF mice exhibited reduced cardiac fibrosis without changes in hypertrophy. This reduction was accompanied by increased cardiac expression of SIRT3. Upregulation of several downstream mitochondrial targets of SIRT3 was also observed. These included mitochondrial fission protein 1 (Fis1), a critical regulator of mitochondrial dynamics, and the antioxidant enzyme heme oxygenase-1 (Hmox1). In contrast, levels of hydroxy-3-methylglutaryl coenzyme A (CoA) synthase 2 (HMGCS2) were decreased, while both 3-hydroxybutyrate dehydrogenase 1 (Bdh1) and succinyl-CoA:3-ketoacid CoA transferase (Oxct1) were elevated. Furthermore, genes involved in the electron transport chain, such as ubiquinol-cytochrome C reductase hinge protein (Uqcrh, Complex III) and mitochondrially encoded cytochrome c oxidase I (Mt-Co1, Complex IV), were upregulated.
Discussion: Distinct alterations in cardiac mitochondrial function were observed depending on the presence or absence of IR in obese HFpEF mice. These findings suggest that SIRT3 may play a central role in mediating mitochondrial adaptations in the heart and could represent a promising therapeutic target in HFpEF.
Keywords: HFPEF; SIRT3; insulin resistance; mitochondria metabolism; obesity.
© 2025 Valero-Muñoz, Cooper, Li, Saw, Wilson, Kusminski, Scherer and Sam.
Conflict of interest statement
FS is a full-time employee of Eli Lilly and Co, Indianapolis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. Br Med J. (2000) 321(7255):215–8. 10.1136/bmj.321.7255.215 - DOI - PMC - PubMed
-
- Romero Funes D, Gutierrez Blanco D, Botero-Fonnegra C, Hong L, Lo Menzo E, Szomstein S, et al. Bariatric surgery decreases the number of future hospital admissions for diastolic heart failure in subjects with severe obesity: a retrospective analysis of the US national inpatient sample database. Surg Obes Relat Dis. (2022) 18(1):1–8. 10.1016/j.soard.2021.09.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

