Smad3 Mediates Renal Fibrosis via GPX4-Dependent Ferroptosis
- PMID: 41079940
- PMCID: PMC12509913
- DOI: 10.7150/ijbs.114075
Smad3 Mediates Renal Fibrosis via GPX4-Dependent Ferroptosis
Abstract
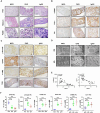
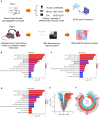
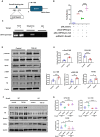
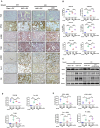
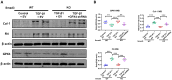
TGF-β/Smad3 signaling is a key pathway leading to the cell death and renal fibrosis. Here we report a new mechanism through which Smad3 mediates renal fibrosis by downregulating the glutathione peroxidase 4 (GPX4), a central inhibitor for ferroptosis. In patients with chronic kidney disease (CKD) and a mouse model of unilateral ureteral obstruction (UUO), progressive renal fibrosis was associated with the overactive Smad3 signaling and the development of ferroptosis identified by decreased GPX4 while increasing two ferroptosis biomarkers including the Transferrin receptor 1 (TFR1) and 4-Hydroxynonenal (4-HNE). Mechanistically, we uncovered that Smad3 could bind directly to GPX4 to repress its transcription while increasing TFR1 and 4-HNE expression, which was abolished when this binding site was mutated. This novel finding was functionally confirmed in the UUO mice and mouse embryonic fibroblasts (MEFs) in which deletion of Smad3 protected against UUO and transforming growth factor-β1 (TGF-β1)-induced loss of GPX4, upregulation of TFR1 and 4-HNE, and progressive renal fibrosis in vivo and in vitro. Importantly, we also found that GPX4 was a downstream target gene of Smad3 and functioned to protect against Smad3-mediated renal fibrosis as silencing GPX4 restored UUO-induced severe renal fibrosis in Smad3 KO mice and in TGF-β1-stimulated Smad3 KO MEFs and SIS3-treated HK-2 cells. Thus, GPX4 is protective in renal fibrosis. Smad3 mediates renal fibrosis via a mechanism associated with GPX4-dependent ferroptosis. The protective effect of GPX4 on Smad3-mediated renal pathologies suggests that targeting the Smad3/GPX4 axis may be a novel therapy for CKD.
Keywords: Ferroptosis; GPX4; Renal fibrosis; Smad3; TGF-β1..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Nastase MV, Zeng-Brouwers J, Wygrecka M, Schaefer L. Targeting renal fibrosis: Mechanisms and drug delivery systems. Adv Drug Deliv Rev. 2018;129:295–307. - PubMed
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–52. - PubMed
-
- Loeffler I, Wolf G. Transforming growth factor-beta and the progression of renal disease. Nephrol Dial Transplant. 2014;29(Suppl 1):i37–i45. - PubMed
-
- Liu Y. Kidney Fibrosis: Fundamental Questions, Challenges, and Perspectives. Integrative Medicine in Nephrology and Andrology. 2024;11(4):e24–00027.
-
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

