Long-term Survival Analysis From PERLA, A Phase II Randomized Trial of Dostarlimab With Chemotherapy Versus Pembrolizumab With Chemotherapy in Metastatic Nonsquamous NSCLC
- PMID: 41080090
- PMCID: PMC12509971
- DOI: 10.1016/j.jtocrr.2025.100900
Long-term Survival Analysis From PERLA, A Phase II Randomized Trial of Dostarlimab With Chemotherapy Versus Pembrolizumab With Chemotherapy in Metastatic Nonsquamous NSCLC
Abstract
Introduction: PERLA is a global, double-blind, phase II trial comparing anti-programmed cell death protein 1 antibodies, dostarlimab, and pembrolizumab in combination with chemotherapy (D+CT and P+CT, respectively) in patients with metastatic nonsquamous NSCLC without actionable genomic aberrations in the first-line setting.
Methods: Patients were randomized 1:1 to receive not more than 35 cycles of 500 mg dostarlimab or 200 mg pembrolizumab, with less than or equal to 35 cycles of 500 mg/m2 pemetrexed and less than or equal to 4 cycles of cisplatin (75 mg/m2) or carboplatin (area under the curve 5 mg/mL/min) every 3 weeks. The primary end point was the overall response rate by blinded independent central review. The secondary end points included progression-free survival (PFS) on the basis of investigator assessment, overall survival (OS), and safety. Here, we reported on the long-term OS, PFS, and safety analyses.
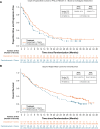
Results: At the end of the study (September 10, 2024), the median follow-up time (mo) for PFS was 30.4 for D+CT and 30.4 for P+CT. The median PFS (mo [95% confidence interval (CI)]) was 8.8 (6.9-11.0) for D+CT and 6.8 (4.9-7.1) for P+CT (hazard ratio 0.77 [95% CI: 0.58-1.03] at 79% maturity). The median follow-up time (mo) for OS was 35.5 for D+CT and 35.2 for P+CT. The median OS (mo [95% CI]) was 20.2 (14.5-27.3) and 15.9 (11.6-19.3), respectively (hazard ratio 0.75 [95% CI: 0.55-1.02] at 70% maturity). Safety profiles were similar between arms and consistent with previous analyses.
Conclusions: This long-term analysis reaffirms previous observations that D+CT exhibited similar efficacy to P+CT and exhibits strong clinical efficacy as a first-line treatment for patients with metastatic nonsquamous NSCLC.
Clinical trial registration: NCT04581824.
Keywords: Dostarlimab; Immune checkpoint inhibitors; NSCLC; PD-1 receptor; Pembrolizumab.
© 2025 The Authors.
Conflict of interest statement
Dr. Lim has received research grants from Yuhan and Johnson and Johnson; received consulting fees from AstraZeneca, Boehringer Ingelheim, Lilly, Takeda, Guardant, J Ints Bio, Therapex, Ono BMS; and has been an investigator for clinical trials sponsored by AstraZeneca, BeiGene, Boehringer Ingelheim, GSK, Roche, Hengrui, BridgeBio Therapeutics, Oscotec, Daichii Sankyo, Amgen, Therapex, Yuhan, Johnson and Johnson, and Takeda. Dr. Ortega-Granados is an employee of the Servicio Andaluz de Salud and has had an advisory role for Roche, Bristol Myers Squibb, and Merck Sharp and Dohme. Dr. Pinto has been an invited speaker for AstraZeneca and Daiichi Sankyo. Dr. Fuentes has been an invited speaker for Fundacion Respirar. Dr. Lo Russo has received consulting fees from Regeneron, Lilly, Roche, Novartis, BMS, MSD, AstraZeneca, Takeda, Amgen, Sanofi, Johnson and Johnson, Merck, Pierre Fabre, Bayer, Beigene, Daiichi, GSK, and Pfizer; received honoraria from Roche, Novartis, BMS, MSD, AstraZeneca, Takeda, Amgen, and Sanofi; received travel grants from Roche, BMS, and MSD; had an advisory role for Roche, Novartis, BMS, MSD, AstraZeneca, and Sanofi; and has been an investigator for clinical trials sponsored by Roche, Novartis, BMS, MSD, AstraZeneca, GSK, Amgen, and Sanofi. Dr. Schenker has had contracts for clinical trial activities (institutional and personal as site Principal Investigator) with GSK, Merck Serono, BMS, MSD, Roche, Sanofi, Regeneron, Astellas, Amgen, Bayer, BeiGene, Clovis, Tesaro, Gilead, Bioven, Novartis, Pfizer, Eli Lilly, Pharma Mar, AbbVie, AstraZeneca, Mylan, and Daiichi Sankyo. Dr. Ahn has been an invited speaker for Boryung, LG Chemical, Nokwon Medical, Samyang, Lilly Korea, Kyowa Kirin, Amgen Korea, Yuhan, AstraZeneca Korea, Menarini Korea, Bayer Korea, Takeda Pharmaceutical, Novartis Korea, BC World, Pfizer, Roche Korea, and Boehringer Ingelheim; and had an advisory role for Immuneoncia, Daiichi Sankyo Korea, Pfizer, Yuhan, Pharmbio Korea, Roche, Therapex, and Guardant. Dr. de Marinis has had an advisory role for AstraZeneca, Roche, Novartis, Merck, BMS, and MSD. Dr. Locke Jr and Dr. Szijgyarto are employed by GSK. Ms. Buss, Dr. Stjepanovic, and Dr. Diaz-Padilla are employed by GSK and hold financial equities in GSK. Dr. Peters has an advisory role with AbbVie, AiCME, Amgen, Arcus, AstraZeneca, Bayer, Beigene, Biocartis, BioInvent, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, ecancer, Eli Lilly, Elsevier, F-Star, Fishawack, Foundation Medicine, Genzyme, Gilead, GSK, Illumina, Imedex, IQVIA, Incyte, iTeos, Janssen, Medscape, Medtoday, Merck Sharp and Dohme, Merck Serono, Merrimack, Novartis, Novocure, Oncology Education, Pharma Mar, Phosplatin Therapeutics, PER, Peerview, Pfizer, PRIME, Regeneron, RMEI, Roche/Genentech, RTP, Sanofi, Seattle Genetics, Takeda, and Vaccibody; has been an invited speaker for AiCME, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, eCancer, Eli Lilly, Foundation Medicine, Illumina, Imedex, Medscape, Merck Sharp and Dohme, Mirati, Novartis, PER, Peerview, Pfizer, Prime, Roche/Genentech, RTP, Sanofi, and Takeda; and received research grants from Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb, GSK, Merck Sharp and Dohme, and Roche/Genentech.
Figures
References
-
- American Cancer Society Cancer facts & figures. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts...
-
- GSK LLC JEMPERLI (dostarlimab) https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761174s010lbl.pdf
-
- Peters S., Ortega Granados A.L., de Marinis F., et al. LBA64 overall survival from a phase II randomised double-blind trial (PERLA) of dostarlimab (dostar) + chemotherapy (CT) vs pembrolizumab (pembro) + CT in metastatic non-squamous NSCLC. Ann Oncol. 2023;34
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

