Machine learning risk stratification strategy for multiple myeloma: Insights from the EMN-HARMONY Alliance platform
- PMID: 41080508
- PMCID: PMC12509237
- DOI: 10.1002/hem3.70228
Machine learning risk stratification strategy for multiple myeloma: Insights from the EMN-HARMONY Alliance platform
Abstract
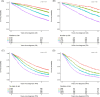
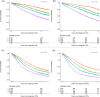
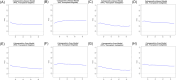
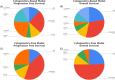
Traditional risk stratification in multiple myeloma (MM) relies on clinical and cytogenetic parameters but has limited predictive accuracy. Machine learning (ML) offers a novel approach by leveraging large datasets and complex variable interactions. This study aimed to develop and validate novel ML-driven prognostic scores for newly diagnosed MM (NDMM), with the goal of improving upon existing ones. To this end, we analyzed data from the EMN-HARMONY MM cohort, comprising 14,345 patients, including 10,843 NDMM patients enrolled across 16 clinical trials. Three ML models were developed: (1) a comprehensive model incorporating 20 variables, (2) a reduced model including six key variables (age, hemoglobin, β2-microglobulin, albumin, 1q gain, and 17p deletion), and (3) a cytogenetics-free model. All models were internally validated using out-of-bag cross-validation and externally validated with data from the Myeloma XI trial. Model performance was evaluated using the concordance index (C-index) and time-dependent area under the receiver operating characteristic curve (ROC-AUC). The comprehensive model achieved C-index values of 0.666 (training) and 0.667 (test) for overall survival (OS) and 0.620/0.627 for progression-free survival (PFS). The reduced model maintained accuracy (OS: 0.658/0.657; PFS: 0.608/0.614). The cytogenetics-free model showed C-index values of 0.636/0.643 for OS and 0.600/0.610 for PFS. Incorporating treatment type and best response to first-line treatment further improved performance. The new prognostic models improved over the International Staging System (ISS), Revised International Staging System (R-ISS), and Second Revision of the International Staging System (R2-ISS) and were reproducible in real-world and relapsed/refractory MM, including daratumumab-treated patients. This ML-based risk stratification strategy provides individualized risk predictions, surpassing traditional group-based methods and demonstrating broad applicability across patient subgroups. An online calculator is available at https://taxonomy.harmony-platform.eu/riskcalculator/.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Adrian Mosquera Orgueira: Roche: consultancy; Pfizer: consultancy; AbbVie: membership on an entity's board of directors or advisory committees, speakers bureau; AstraZeneca: consultancy, membership on an entity's board of directors or advisory committees, speakers bureau; Janssen: consultancy, membership on an entity's board of directors or advisory committees, speakers bureau; Takeda: speakers bureau; Biodigital THX: current equity holder in private company; Novartis: other; Incyte: other; GSK: consultancy. Mattia D'Agostino: GlaxoSmithKline: honoraria, membership on an entity's board of directors or advisory committees; Sanofi: honoraria, membership on an entity's board of directors or advisory committees; Bristol Myers Squibb: membership on an entity's board of directors or advisory committees; Janssen: honoraria, research funding; Adaptive Biotechnologies: membership on an entity's board of directors or advisory committees. Alessandra Larocca: Janssen: honoraria, other: participation in advisory board; GSK: honoraria, other: participation in advisory board; Menarini: honoraria, other: participation in advisory board; Sanofi: honoraria. Ruth Wester: Sanofi: honoraria; Janssen: honoraria. Hans Salwender: Janssen: honoraria, other: travel grant; Oncopeptides: honoraria; Pfizer: honoraria; Sanofi: honoraria, other: travel grant; Stemline: honoraria; Roche: honoraria; Takeda: honoraria; Chugai: honoraria; GlaxoSmithKline: honoraria; Bristol Myers Squibb/Celgene: honoraria, other: travel grant; Amgen: honoraria, other: travel grant; AbbVie: honoraria; Sebia: honoraria. Sara Bringhen: AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, and Sanofi: speakers bureau; Sanofi: consultancy, honoraria; Bristol Myers Squibb, Janssen, Oncopeptides, Pfizer, Stemline Therapeutics, and Takeda: other: participation in advisory boards. Sonja Zweegman: Sanofi: membership on an entity's board of directors or advisory committees; BMS: membership on an entity's board of directors or advisory committees; GSK: membership on an entity's board of directors or advisory committees; Janssen: membership on an entity's board of directors or advisory committees, research funding; Amgen: membership on an entity's board of directors or advisory committees; Takeda: research funding; Oncopeptides: membership on an entity's board of directors or advisory committees. Jesus Maria Hernandez Rivas: GlaxoSmithKline: consultancy, honoraria; Amgen: honoraria, membership on an entity's board of directors or advisory committees, speakers bureau; Pfizer: honoraria, membership on an entity's board of directors or advisory committees; Bristol Myers Squibb: honoraria, membership on an entity's board of directors or advisory committees, research funding, speakers bureau. Gordon Cook: Celgene: research funding; Amgen: consultancy, speakers bureau; Janssen: consultancy, research funding; Bristol Myers Squibb: consultancy, honoraria; Janssen‐Cilag: honoraria, speakers bureau; Takeda: consultancy, honoraria, research funding, speakers bureau. Martin F. Kaiser: Pfizer: consultancy, honoraria; GSK: consultancy; Sanofi: consultancy; BMS/Celgene: consultancy, honoraria, research funding; Pfizer: consultancy, honoraria; J&J/Janssen: consultancy, honoraria, research funding; Roche: consultancy; Poolbeg: consultancy, honoraria; Regeneron: consultancy. Hartmut Goldschmidt: Sanofi: honoraria, membership on an entity's board of directors or advisory committees, other: grants and/or provision of investigational medicinal product; support for attending meetings and/or travel, research funding; Chugai: honoraria, other: grants and/or provision of investigational medicinal product; Adaptive Biotechnologies: membership on an entity's board of directors or advisory committees; Millennium Pharmaceuticals Inc.: research funding; Takeda: research funding; Amgen: honoraria, membership on an entity's board of directors or advisory committees, other: support for attending meetings and/or travel; grants and/or provision of investigational medicinal product, research funding; Celgene: research funding; Bristol Myers Squibb: honoraria, membership on an entity's board of directors or advisory committees, other: support for attending meetings and/or travel, research funding; Janssen: honoraria, membership on an entity's board of directors or advisory committees, other: grants and/or provision of investigational medicinal product; support for attending meetings and/or travel, research funding; Karyopharm: research funding; Hoffmann‐La Roche: research funding; Molecular Partners: research funding; Novartis: honoraria, other: support for attending meetings and/or travel, research funding; MorphoSys AG: research funding; Bristol Myers Squibb/Celgene: other: grants and/or provision of investigational medicinal product; Merck Sharp and Dohme (MSD): research funding; GlycoMimetics Inc.: research funding; Incyte Corporation: research funding; Dietmar Hopp Foundation: other: grants and/or provision of investigational medicinal product; Array Biopharma/Pfizer: other: grants and/or provision of investigational medicinal product; GlaxoSmithKline (GSK): honoraria, other: support for attending meetings and/or travel, research funding; Heidelberg Pharma: research funding; Pfizer: honoraria, other: support for attending meetings and/or travel, research funding; Johns Hopkins University: other: grants and/or provision of investigational medicinal product; Mundipharma GmbH: other: grants and/or provision of investigational medicinal product. Pieter Sonneveld: Oncopeptides: patents and royalties; Karyopharm: membership on an entity's board of directors or advisory committees, patents and royalties, research funding; Pfizer: membership on an entity's board of directors or advisory committees, patents and royalties; European Myeloma Network: other: president; Celgene: membership on an entity's board of directors or advisory committees, research funding; Janssen: membership on an entity's board of directors or advisory committees, patents & royalties, research funding; Bristol Myers Squibb: membership on an entity's board of directors or advisory committees, research funding; Amgen: membership on an entity's board of directors or advisory committees, research funding. Jesús F. San‐Miguel: Bristol Myers Squibb: other: advisory board; Celgene: other: advisory board; Roche: other: advisory board; Janssen‐Cilag: other: advisory board; Novartis: other; Karyopharm: other: advisory board; GlaxoSmithKline: other: advisory board; Haemalogix: other: advisory board; MSD: other: advisory board; Amgen: consultancy, other: advisory board; Takeda: other: advisory board; Sanofi: other: advisory board; AbbVie: consultancy, other: advisory board; Regeneron: other: advisory board; SecuraBio: other: advisory board. Mario Boccadoro: GlaxoSmithKline: membership on an entity's board of directors or advisory committees; AbbVie: honoraria; Bristol Myers Squibb: honoraria, research funding; Janssen: honoraria, membership on an entity's board of directors or advisory committees, research funding; Novartis: honoraria, research funding; Amgen: honoraria, research funding; Celgene: honoraria, research funding; Sanofi: honoraria, research funding; Mundipharma: research funding. Maria‐Victoria Mateos: BMS/Celgene, Janssen‐Cilag, Sanofi, AbbVie, Stemline, Oncopeptides, GSK: honoraria, membership on an entity's board of directors or advisory committees; Amgen, Takeda, Regeneron: honoraria.
Figures

References
-
- Kumar S, Medeiros LJ, Gujral S, et al. Plasma cell myeloma/multiple myeloma. In: Haematolymphoid Tumours. WHO Classification of Tumours. Vol 11, 5th ed. International Agency for Research on Cancer (IARC). Accessed February 15, 2025. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-O...
LinkOut - more resources
Full Text Sources
