Multifunctional nanoplatform as nano-inducer of ferroptosis for targeted recognition and imaging-guided therapy of metastatic prostate cancer
- PMID: 41080736
- PMCID: PMC12510038
- DOI: 10.1016/j.mtbio.2025.102317
Multifunctional nanoplatform as nano-inducer of ferroptosis for targeted recognition and imaging-guided therapy of metastatic prostate cancer
Abstract
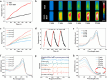
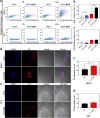
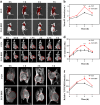
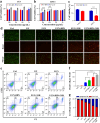
Metastasis prostate cancer (PCa) precision detection and effective treatment remain significant challenge in clinic. Ferroptosis brought promising therapeutic strategy for the treatment of metastatic PCa, effectively inducing ferroptosis in PCa cells represents key to improve therapeutic efficacy. Herein, we developed a multifunctional nanoplatform Fe/Au nanodots-bombesin (FGN-BBN) as the ferroptosis nano-inducer to generate large amount of ROS to induce ferroptosis through an "open-source throttling" strategy for targeted imaging-guided therapy of metastatic PCa. On the one hand, FGN-BBN serves as an efficient biomimetic nanozyme and photothermal agent, exhibiting great POD-like activity and generating abundant reactive oxygen species (ROS) via photothermal-enhanced chemodynamic therapy (CDT) to induce ferroptosis, which is achieving "open source" aspect. On the other hand, FGN-BBN exhibit GPx-like activity that depletes overexpressed glutathione (GSH) within the tumor microenvironment, thereby preventing the neutralization of ROS and achieving the "throttling" effect. Furthermore, bombesin facilitates targeted delivery of the nanozyme to metastatic PCa cells, synergistically enhancing ferroptosis activity. In terms of diagnosis, FGN-BBN possesses targeted recognition capabilities and enables multimode bioimaging including fluorescence (FL), computed tomography (CT), and magnetic resonance imaging (MRI), allowing for the "visualization" of tumor localization and real-time imaging-guided therapy. In summary, the multifunctional nanoplatform integrates multienzyme activity, targeted recognition, multimodal imaging, photothermal therapy, and CDT to induce high-efficiency ferroptosis, offering an effective theranostic strategy for metastatic PCa.
Keywords: Ferroptosis nano-inducer; Metastasis prostate cancer; Multi-mode imaging; Nanozyme; Tumor targeting.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Li S., Kang Y., Zeng Y. Targeting tumor and bone microenvironment: novel therapeutic opportunities for castration-resistant prostate cancer patients with bone metastasis. Biochim. Biophys. Acta Rev. Canc. 2024;1879(1) - PubMed
