Synthesis and characterization of a functional benzoylecgonine - bovine serum albumin conjugate for quantification of a humanized monoclonal anti-cocaine antibody
- PMID: 41080745
- PMCID: PMC12510059
- DOI: 10.1016/j.bbrep.2025.102282
Synthesis and characterization of a functional benzoylecgonine - bovine serum albumin conjugate for quantification of a humanized monoclonal anti-cocaine antibody
Abstract
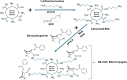
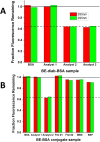
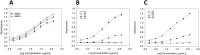
We have developed a humanized monoclonal anti-cocaine antibody (h2E2), as a potential solution for cocaine use disorder. A key milestone was the development of an assay to quantify this monoclonal antibody (mAb) in animal and human blood. Thus, we synthesized a novel benzoylecgonine-1,4-diaminobutane-BSA (BE-diab BSA) conjugate as an antigen for quantifying h2E2 using ELISA. We report here the method of synthesis of this conjugate, BE-diab BSA, and assessment of its binding to the h2E2 mAb using an ELISA and a fluorescence quenching assay. Compared with four commercial BE-BSA conjugates, BE-diab BSA demonstrated markedly stronger mAb binding in ELISA-three of the commercial conjugates showed less than 10 % relative binding. Fluorescence quenching assays confirmed this binding superiority, with the commercial conjugates showing minimal mAb interaction, while BE-diab BSA induced robust intrinsic fluorescence quenching. SDS-PAGE analyses identified structural differences consistent with binding results between our functional conjugate and the commercial preparations. This functional and reproducible in-house conjugation has been integrated into a GLP-validated ELISA, which is now in use for pharmacokinetic analyses and for qualifying antibody release lots for clinical deployment.
Keywords: Anti-cocaine antibody; Antigen-synthesis; BE-diab BSA; Cocaine; ELISA; Humanized; Monoclonal.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Andrew B. Norman reports financial support was provided by 10.13039/100000026National Institute on Drug Abuse. Andrew B. Norman has patent #10501556 issued to University of Cincinnati, E. R. Squibb & Sons, L.L.C. Andrew B. Norman has patent #9957332 issued to University of Cincinnati. Andrew B. Norman has patent #9758593 issued to University of Cincinnati, E. R. Squibb & Sons, L.L.C.
Figures


References
-
- Frank J., Vocci PhD., Jane Acri PhD., Ahmed Elkashef M.D. Medication development for addictive disorders: the state of the science. Am. J. Psychiatr. 2005;162:1432–1440. - PubMed
-
- Paula S., Tabet M.R., Farr C.D., Norman A.B., Ball W.J., Jr. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J. Med. Chem. 2004;47:133–142. - PubMed
LinkOut - more resources
Full Text Sources

