Evaluating the Effect on Total Daily Insulin Dose in Adult Patients with Type 1 Diabetes Managed with Metformin and/or GLP-1 or GLP-1/GIP Receptor Agonists
- PMID: 41080834
- PMCID: PMC12509713
- DOI: 10.24926/iip.v16i1.6450
Evaluating the Effect on Total Daily Insulin Dose in Adult Patients with Type 1 Diabetes Managed with Metformin and/or GLP-1 or GLP-1/GIP Receptor Agonists
Abstract
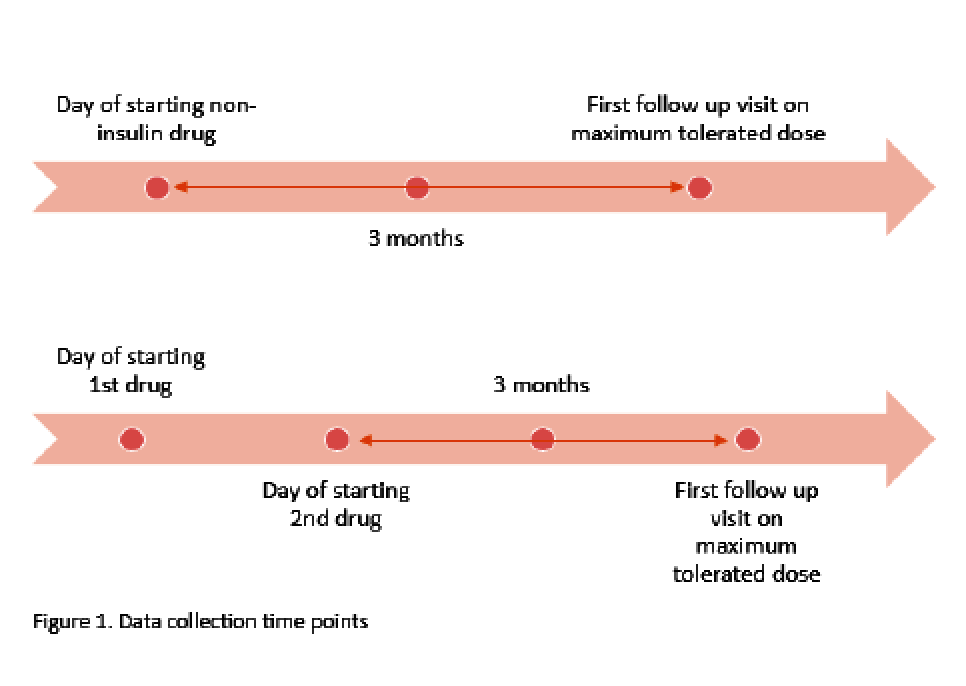
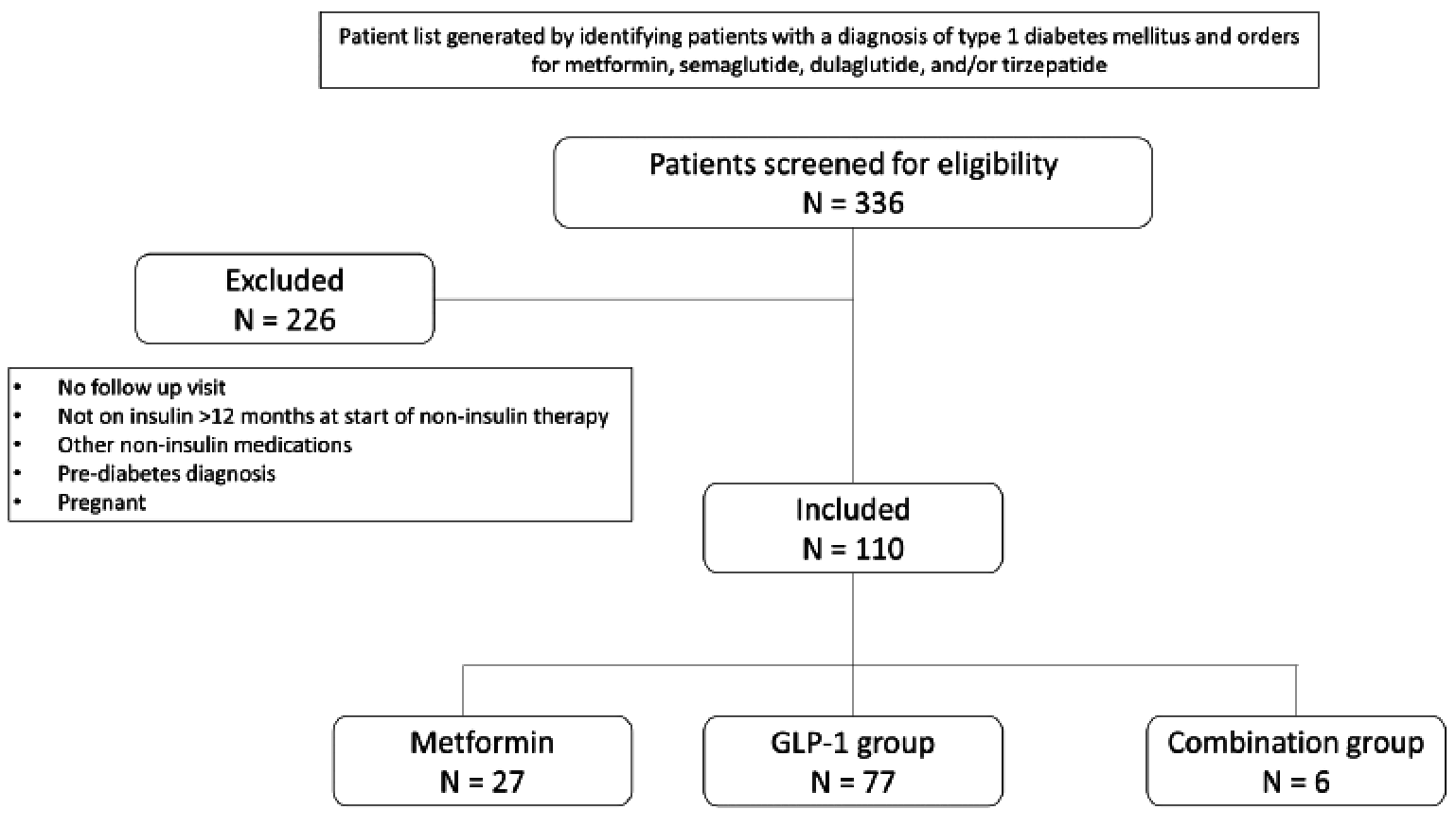
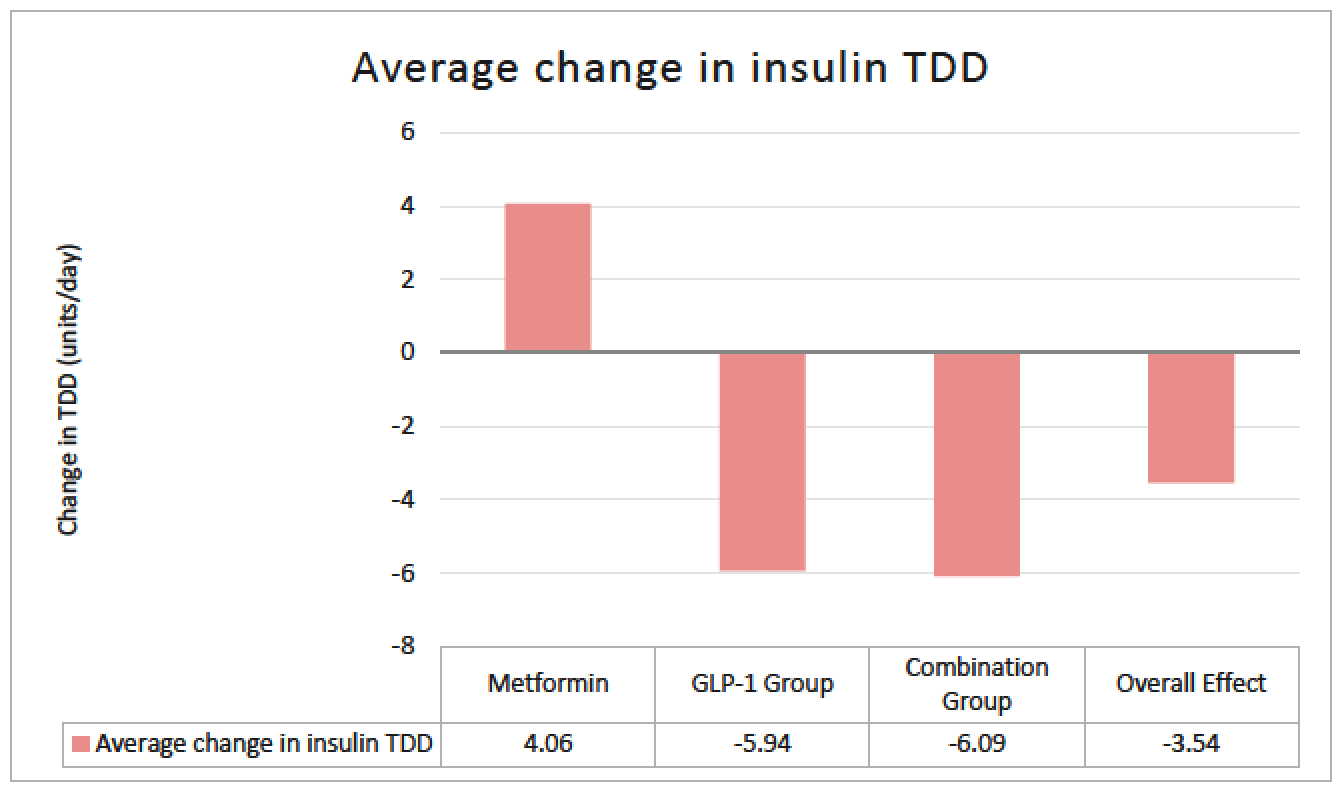
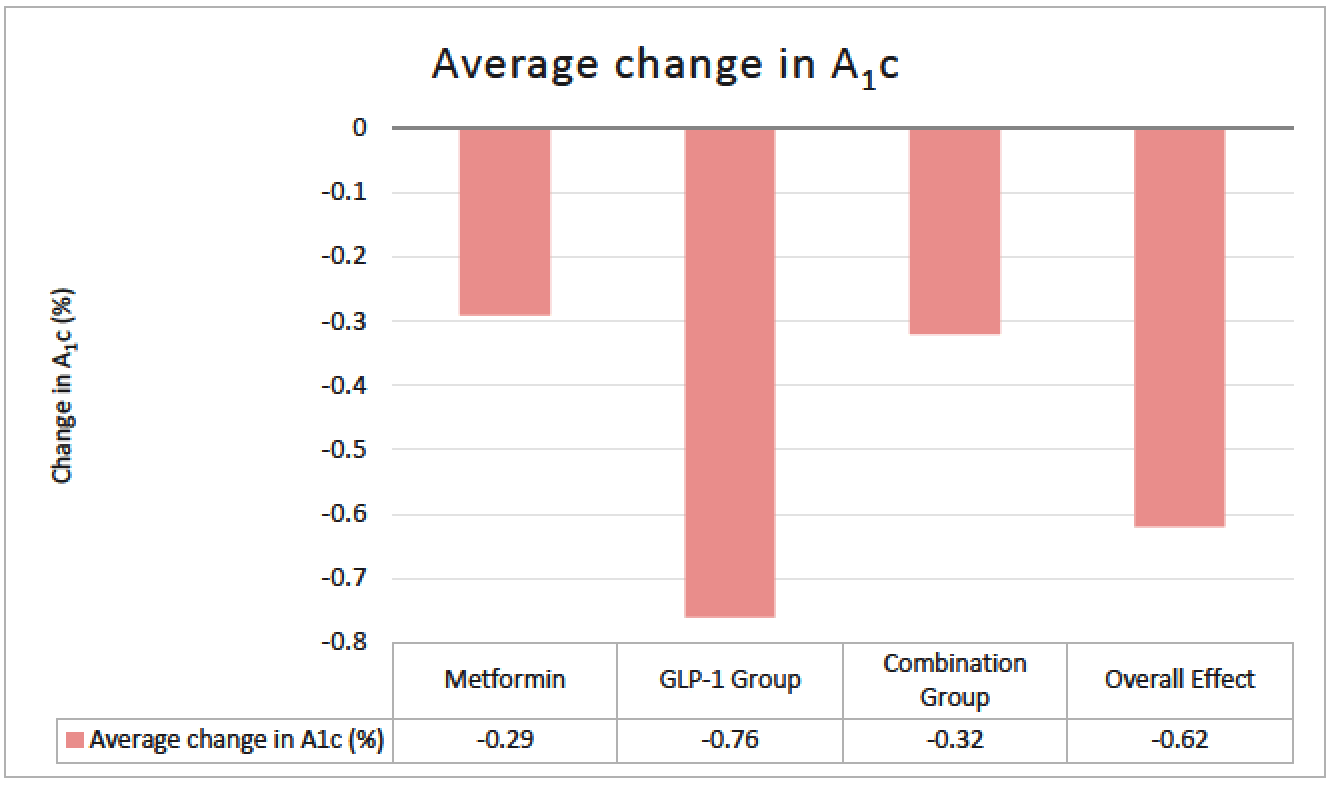
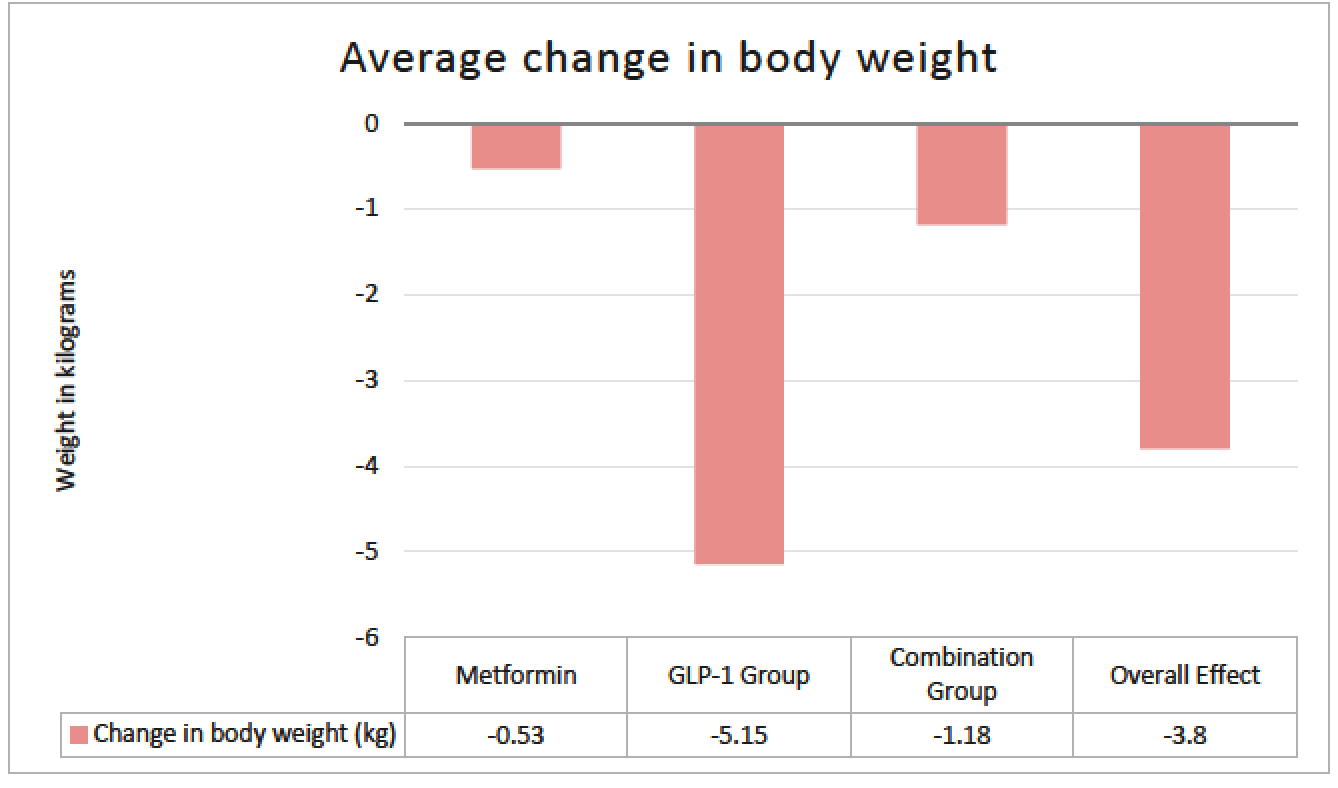
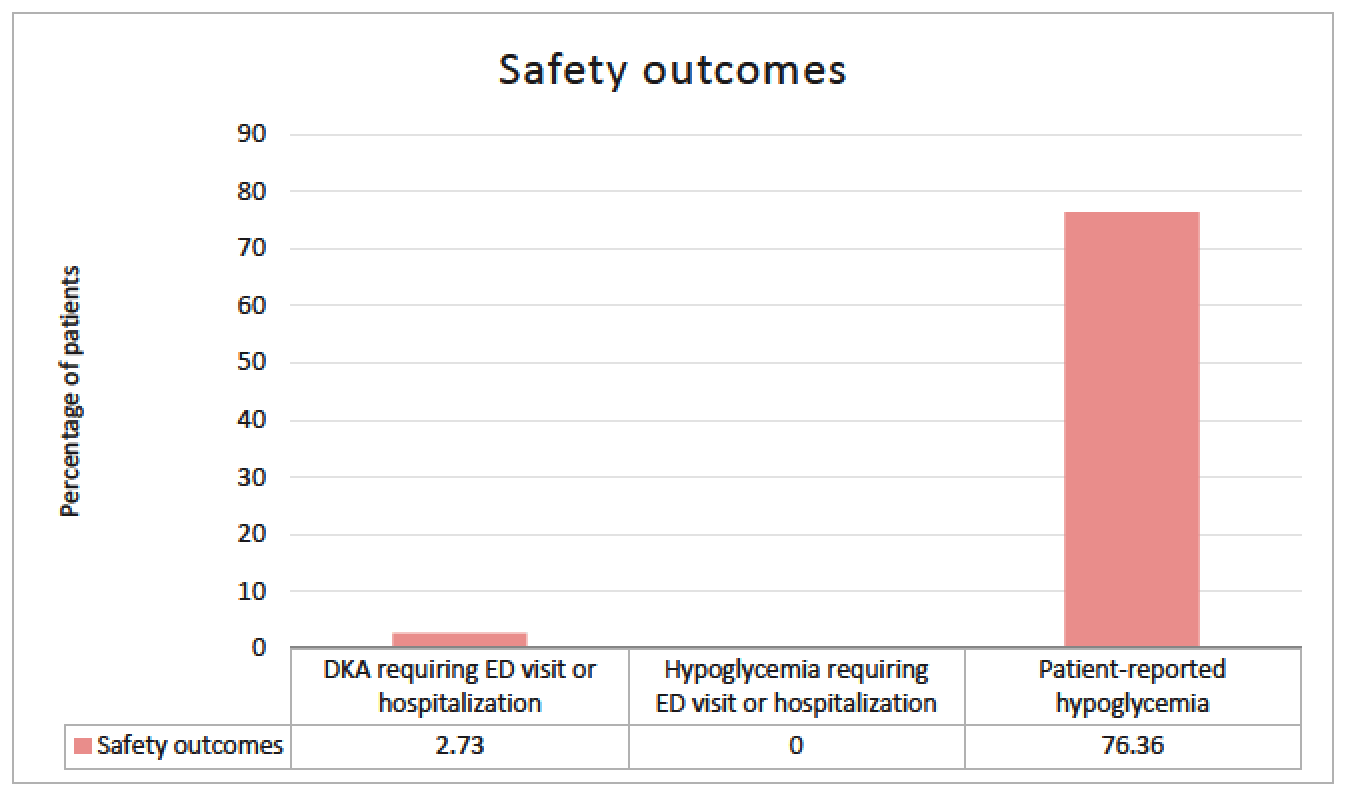
Purpose: There are few studies that have assessed the utility of metformin and glucagon-like peptide-1 receptor agonists (GLP-1 RA) in type 1 diabetes (T1D), specifically looking at glucose control indices. These studies have largely evaluated the impact of agents within the class that are not routinely used. Limited data exist on the use of the dual glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide receptor agonist (GLP-1/GIP RA) in T1D. The objective of this study was to evaluate the effect of this growing practice in utilizing these common non-insulin therapies in T1D. Methods: This single-center, retrospective cohort study evaluated adult patients with T1D who received standard insulin therapy plus the following non-insulin therapies for at least 3 months: metformin; GLP-1 RA or GLP-1/GIP RA; or metformin and a GLP-1 RA or GLP-1/GIP RA (combination group). Data points were collected on starting dates of the first and second (if applicable) non-insulin agents, and the first office visit of at least 3 months on maximum tolerated doses. The primary endpoint was change in total daily insulin dose (TDD). Secondary and safety endpoints were evaluated in A1c, weight, and hypoglycemia. Results: A total of 110 of 366 patients met inclusion criteria. Changes in average insulin TDD were +4.06, -5.9 and -6.9 units for the metformin, GLP-1RA or GLP-1/GIP RA, and combination groups respectively (P =0.013). TDD after non-insulin therapy addition decreased in all patients on average 3.54 units (P =0.02). Non-insulin therapies showed a significant decrease in A1c by 0.62%, weight by 3.8kg, and hypoglycemia was seen in 76% of patients. Conclusions: Non-insulin therapies added to standard insulin therapy in T1D resulted in decreased insulin requirement, increased glycemic control, and decreased body weight. While statistically significant, it remains unclear if the decreased insulin requirement is clinically significant. Further prospective studies are warranted to validate these findings.
Keywords: GLP-1/GIP receptor agonists; glucagon-like peptide-1 receptor agonists; glycemic control; metformin; non-insulin therapy; type 1 diabetes.
© Individual authors.
Figures
References
-
- Lucier J, Weinstock RS.. Type 1 Diabetes (updated 2023). www.ncbi.nlm.nih.gov/books/NBK507713/ (accessed 2023 September 1).
-
- American Diabetes Association. The History of A Wonderful Thing We Call Insulin (2019) www.diabetes.org/blog/history-wonderful-thing-we-call-insulin (accessed 2023 September 1).
-
- American Diabetes Association. Diabetes Care. Standards of Care in Diabetes (2024). www.diabetesjournals.org/care/issue/47/Supplement_1 (accessed 2024 April 20).
-
- Ahrén B, Hirsch IB, Pieber TR, et al. ; ADJUNCT TWO Investigators. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care 2016; 39:1693–1701. - PubMed
-
- Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016; 4:221–232. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
