Alpha-toxin-elicited CX3CL1 release in Staphylococcus aureus pneumonia impairs bactericidal function of human monocytes
- PMID: 41081526
- PMCID: PMC12607776
- DOI: 10.1128/mbio.02689-25
Alpha-toxin-elicited CX3CL1 release in Staphylococcus aureus pneumonia impairs bactericidal function of human monocytes
Abstract
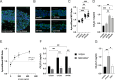
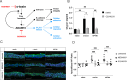
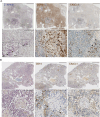
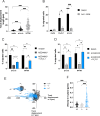
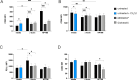
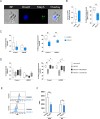
Staphylococcus aureus is an important human pathogen causing severe invasive infections. Pathogenesis is attributed to a wide array of virulence factors, including several potent exotoxins such as the pore-forming α-toxin. In this study, we found that patients with S. aureus respiratory tract infections had elevated CX3CL1 levels in airway fluid and plasma. Using human-organotypic lung models, we observed that stimulation of lung epithelium with α-toxin induces an intensified CX3CL1 expression apically in the epithelium as well as the release of CX3CL1. Blocking α-toxin or ADAM10 activity in organotypic lung using an α-toxin-blocking antibody or a specific ADAM10 inhibitor confirmed their role in modulating CX3CL1 cleavage and release. Analyses of CD14+ human monocytes in combination with a CX3CR1 inhibitor revealed that α-toxin-mediated CX3CL1 release induces CX3CL1-dependent chemotaxis. In line with these data, lung tissue from patients with S. aureus respiratory tract infection showed elevated CX3CL1 and CD14 staining as compared with tissue from patients with non-infectious lung diseases. Functional studies of monocytes showed that CX3CL1 released by lung models resulted in upregulated CD83 and downregulated CD86, as well as impaired killing of phagocytosed S. aureus. Furthermore, stimulation of monocytes with soluble CX3CL1 hampered their reactive-oxygen and nitric-oxide production. Taken together our data show that S. aureus triggers the release of lung epithelial CX3CL1, and we identify an immunomodulatory effect of α-toxin involving its cytotoxic and ADAM10-interacting properties, inducing CX3CL1 release leading to impaired monocyte effector function.IMPORTANCEExotoxins are essential virulence factors for the pathobiont S. aureus and contribute toward severe invasive infections such as pneumonia. S. aureus α-toxin is a pore-forming exotoxin that causes host cell lysis and severe lung pathology. We found that α-toxin drives the release of membrane-bound chemokine CX3CL1 by involving ADAM10-mediated proteolytic activity. Furthermore, the release of CX3CL1 modulated immune responses locally, as demonstrated by enhanced monocyte migration and reduced capacity of monocytes to kill ingested bacteria. CX3CL1-induced reduction in bacterial killing coincided with impaired production of reactive oxygen and nitric oxide species. This reveals a novel mechanism in the pathogenesis of S. aureus lung infections involving α-toxin-induced release of CX3CL1, leading to impaired bacterial killing by monocytes.
Keywords: ADAM10; CX3CL1; Staphylococcus aureus; chemotaxis; fractalkine; monocytes; pneumonia; α-toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR, Whitney AR, Otto M, DeLeo FR. 2009. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 199:1698–1706. doi: 10.1086/598967 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
